Abstract
The capacity to survive and thrive in conditions of limited resources and high inflammation is a major driver of tumor malignancy. Here we identified slow-cycling ADAM12+PDGFRα+ mesenchymal stromal cells (MSCs) induced at the tumor margins in mouse models of melanoma, pancreatic cancer and prostate cancer. Using inducible lineage tracing and transcriptomics, we demonstrated that metabolically altered ADAM12+ MSCs induced pathological angiogenesis and immunosuppression by promoting macrophage efferocytosis and polarization through overexpression of genes such as Gas6, Lgals3 and Csf1. Genetic depletion of ADAM12+ cells restored a functional tumor vasculature, reduced hypoxia and acidosis and normalized CAFs, inducing infiltration of effector T cells and growth inhibition of melanomas and pancreatic neuroendocrine cancer, in a process dependent on TGF-β. In human cancer, ADAM12 stratifies patients with high levels of hypoxia and innate resistance mechanisms, as well as factors associated with a poor prognosis and drug resistance such as AXL. Altogether, our data show that depletion of tumor-induced slow-cycling PDGFRα+ MSCs through ADAM12 restores antitumor immunity.
Similar content being viewed by others
Main
Inflammation and hypoxia are hallmarks of invasive tumors and major drivers of tumor progression1,2. The tumor microenvironment (TME) is characterized by poorly functional angiogenic vasculature, inflammatory infiltrates and high levels of tissue remodeling. Impaired blood flow further restricts delivery of oxygen, antibodies and drug delivery. The resulting hypoxia promotes immunosuppression by upregulation of transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), tumor-associated macrophages (TAMs), nutrient deprivation, a switch in tumor metabolism and acidification of the microenvironment3,4. Such an immunosuppressive microenvironment promotes adaptive and invasive mechanisms including cancer cell dormancy, a state of low proliferation that is common in stem cells and stem-like cells5. This altered metabolic state allows survival in conditions of limited resources, protects from antimitotic drugs and promotes metastasis6.
Growth of solid tumors induces a stromal reaction due to local damage, particularly at the tumor margin, a transition zone that is rich in immune cells, blood vessels and mesenchymal cells. Expansion of mesenchymal cells, also called carcinoma-associated fibroblasts (CAFs), around and within a tumor mass is associated with resistance to therapy and poor clinical outcomes7,8. Tumor stromal cells express mesenchymal markers such as podoplanin (PDPN) and platelet-derived growth factor receptor alpha (PDGFR-α), and are highly heterogeneous, as has been shown by single cells RNA sequencing (RNA-seq) studies in several tumor types9,10,11. They express, albeit not specifically, a number of factors and pathways associated with recruitment of immune cells, angiogenesis, myofibroblast activation and extracellular matrix (ECM) remodeling, including fibroblast-activation protein (FAP), CXCL12, Lox, TGF-β and Hedgehog pathways, all of which play a role in tumor progression12,13,14. Broad targeting of CAFs or collagen production has led to mixed results, because stromal cells and the ECM are required for tissue homeostasis and to restrain tumor growth15,16,17,18,19,20,21. As part of the tumor mass, stromal cells also adapt their metabolism, yet the impact on the tumor microenvironment and antitumor immunity in vivo remains unclear.
A disintegrin and metalloprotease 12 (ADAM12) is a membrane-bound metalloprotease that is expressed during organ morphogenesis and is re-induced in mesenchymal cells during repair and fibrosis and in solid tumors, including pancreas, prostate, breast, colon, bladder and liver cancers and melanoma, both in human disease and mouse models22,23,24,25,26,27. ADAM12 overexpression has been associated with resistance to chemotherapy and poor prognosis26,28,29,30,31,32,33; however, the role of ADAM12+ MSCs in tumorigenesis has not been addressed. Here, we use reporter and deleter genetic models to demonstrate that ADAM12+ cells are a subset of slow-cycling PDGFRα+ mesenchymal perivascular cells, distinct from pericytes, that induce angiogenesis and immunosuppression by promoting TAMs efferocytosis and polarization. Genetic depletion of ADAM12+ cells normalizes the tumor vasculature and decreases hypoxia and acidity, restoring antitumor immunity and blocking tumor growth in mouse models of melanoma and neuroendocrine pancreatic cancer. We further provide direct genetic evidence that TGF-β receptor 2 (TGFBR2) signaling in ADAM12+ cells is required for their pro-tumorigenic function. We propose that ADAM12+ MSCs are part of an evolutionarily conserved mechanism initiated by the cytostatic TGF-β and modulated by inflammation to promote tissue repair, in cooperation with macrophages. In the context of tumorigenesis, such a response represents a major brake for antitumor immunity.
Results
Genetic depletion of ADAM12+ MSCs restores tumor immunity
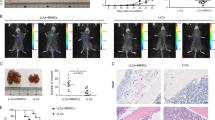
To visualize ADAM12+ cells during tumorigenesis, we subcutaneously inoculated ADAM12-GFP mice)27 with B16-OVA melanoma cells (MO5). We observed that ADAM12+ MSCs (expressing GFP) localized specifically at the tumor margin, a transition zone enriched in stromal cells expressing various levels of PDPN and smooth muscle actin alpha 2 (αSMA+), collagenous ECM, T cells, CD206+ TAMs and blood vessels (Fig. 1a,b and Extended Data Fig. 1a,b). ADAM12–PDPN+PDGFRα+ cells were already abundant in non-tumoral skin (Extended Data Fig. 1c). ADAM12 was not detected in normal skin or in CD45+ tumor immune cells, CD31+ endothelial cells or PDPN–PDGFRα– stromal cells, but its expression was induced in 2–8% of PDGFRα+PDPN+ cells adjacent to peritumoral blood vessels. The frequencies and absolute numbers of ADAM12+ cells decreased at later tumor stages (Fig. 1b,c and Extended Data Fig. 1d–f). ADAM12+ MSCs were PDGFRβ+, αSMA– and NG2lo or NG2– (a pericyte marker), and were localized outside the ColIV+ vascular basement membrane (BM), in contrast to pericytes (Extended Data Fig. 1g–i). To deplete ADAM12+ MSCs, we inoculated ADAM12-DTR mice with MO5 melanoma cells; in these mice, the diphtheria toxin receptor (DTR) is expressed under the control of the Adam12 promotor27. Depletion of ADAM12+ cells starting 10 d after tumor implantation, when tumors were palpable, resulted in 50% inhibition of tumor growth (Fig. 1d and Extended Data Fig. 1l; efficiency of depletion of ADAM12+ cells is shown in ref. 27 and Extended Data Fig. 1j,k). By contrast, depletion of ADAM12+ cells in the initial stages of tumorigenesis did not inhibit tumor growth (Fig. 1e and Extended Data Fig. 1m), arguing against an initial feeder role for stromal cells. Tumors lacking ADAM12+ cells from day 10 had increased infiltration of interferon-γ (IFN-γ)-producing CD8+ T cells and natural killer (NK) cells (Fig. 1f), whereas no difference was observed in infiltration of CD4+ T cells, regulatory T cells (Treg cells), eosinophils, neutrophils, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs) or total macrophages (Extended Data Fig. 1n). We did not measure significant differences in CD8+ T cells or stromal cells in the draining lymph nodes (LNs), consistent with a local effect (Extended Data Fig. 1o,p). Treatment with CD8+ T cell-depleting antibodies restored tumor growth in the absence of ADAM12+ cells, confirming that ADAM12+ cells block antitumor activity of CD8+ T cells (Extended Data Fig. 1q).
a, Immunofluorescence staining of PDPN, αSMA, CD3 and collagen in MO5 melanomas. The inset shows CD3+ T cells (green) on PDPN+ stromal cells (red). b, Immunofluorescence staining of PDPN, CD31 and ADAM12 (GFP) in MO5 melanomas in ADAM12-GFP mice. The inset shows GFP+ cells (green) close to CD31+ blood vessels (blue; marked with an arrowhead). Scale bars, 100 µm. One representative image from four (a) or six (b) independent experiments is shown. Right, FACS plot and percentage of CD45−CD31–cells and GFP+ cells in MO5 melanomas 8 d after tumor inoculation. c, Percentage of GFP+ cells, measured by FACS, in PDGFRα+ stroma isolated from normal skin (day 0, n = 2) or MO5 tumors (n = 6 for 8–12 d and n = 4 for 14–17 d), and in other populations (n = 3). d,e, Tumor growth curves (average tumor volume) from ADAM12-DTR (DTR) and littermate mice (Ctrl) treated with diphteria toxin (DT) from days 10 to 18 (DTR, n = 10; Ctrl, n = 12) (d), or from days 0 to 10 (DTR, n = 5; Ctrl, n = 7) (e) after tumor inoculation. The x axis represents days after tumor inoculation. f, Percentage of tumor-infiltrating CD3+ T cells (n = 8 for Ctrl, n = 12 for DTR), CD8+ T cells (n = 14 for Ctrl, n = 17 for DTR), IFN-γ+CD8+ T cells (n = 7 for Ctrl, n = 6 for DTR), NK cells (n = 9 for Ctrl, n = 9 for DTR) and IFN-γ+ NK cells (n = 16 for Ctrl, n = 14 for DTR) in mice treated with DT from day 10, measured by FACS in three independent experiments. Statistics were calculated using ordinary two-way analysis of variance (ANOVA) (d,e) or two-tailed, unpaired Student’s t-test (f). Quantitative data are presented as means ± s.d. n.s., not significant.
Depletion of ADAM12+ MSCs normalizes the stromal and vascular TME
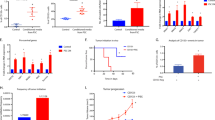
Consistent with an active antitumor immune response, T cells infiltrated the center of tumors depleted of ADAM12+ cells, in proximity to PDPN+ CAFs that had migrated intratumorally and settled near blood vessels (Fig. 2a,b,d). The frequency of T cells in proximity to PDPN+ CAFs was similar in depleted and control conditions (Extended Data Fig. 2a), arguing against PDPN+ CAFs having an increased capability to recruit T cells34. The frequencies and absolute numbers of PDPN+ CAFs were similar in both conditions (Fig. 2c and Extended Data Fig. 2b), suggesting that CAFs became permissive to T cells. To identify changes occurring in PDPN+ CAFs in tumors infiltrated by T cells, we performed RNA-seq gene expression analysis of PDPN+PDGFRα+ cells isolated from wild-type (WT) tumors (which were poorly infiltrated) or from tumors lacking ADAM12+ cells (which were highly infiltrated) (the gating strategy is provided in Extended Data Fig. 9). Differential gene expression analysis in PDPN+PDGFRα+ cells in these two conditions identified a few genes, including Car9, Saa3, Lcn2, Mmp9 and Mmp13, that were significantly downregulated in the CAFs of tumors lacking ADAM12+ cells (DTR versus control) (Fig. 2e). These genes all have well-recognized protumor roles35,36,37,38,39, and their expression is commonly induced by hypoxia. Hypoxia-induced Car9 (carbonic anhydrase 9, CAIX) prevents cytosolic acidification by catalyzing the hydration of carbon dioxide into bicarbonate ions and protons. CAIX expression enhances cell survival in hypoxic conditions and increases acidification of the tumor microenvironment, a major immunosuppressive factor40,41. Consistent with a role for ADAM12+ cells in tumor hypoxia and acidosis, tumors lacking these cells showed decreased hypoxia (Fig. 2f) and increased extracellular pH (Fig. 2g). As oxygen levels were restored in the absence of ADAM12+ cells, Car9 expression was significantly decreased in the total tumor (Fig. 2h). To investigate whether normalization of the pH of the tumor microenvironment alone was sufficient to restore tumor immunity, we treated WT mice bearing MO5 melanomas with the CAIX inhibitor U-104. We observed that, although inhibition of CAIX normalized the extracellular tumor pH to levels similar to those achieved by depletion of ADAM12+ cells (Fig. 2g), antitumor immunity was not restored (Extended Data Fig. 2c). Furthermore, we observed that, in contrast to tumors lacking ADAM12+ cells, U-104-treated tumors were still hypoxic (Extended Data Fig. 2d). Tumor hypoxia results mainly from poorly functional, collapsed tumor vasculature that lacks pericyte coverage, which is required for proper vessel maturation and function42. Consistent with normalization of the vasculature, blood vessels of tumors lacking ADAM12+ cells had restored levels of NG2+ pericyte coverage and increased width (Fig. 2i and Extended Data Fig. 2e), as well as increased expression of ICAM1 (Fig. 2j), which is essential for leukocyte adhesion and trans-endothelial migration, and improved tumor perfusion (Fig. 2k). Of note, genes upregulated in PDPN+PDGFRα+ cells in tumors lacking ADAM12+ cells included regulators of PDGF and BMP signaling (Fig. 2e), also involved in vascular maturation and normalization43,44. Previous single-cell RNA-seq (scRNA-seq) studies of murine melanomas identified three clusters of CAFs, referred to as immune/inflammatory DPP4+CD34hi stroma (S1), desmoplastic stroma (S2) and contractile stroma/pericytes (S3)9. In tumors depleted of ADAM12+ cells, we observed a significant decrease in the frequency of S1 cells, whereas S2 and S3 increased (Extended Data Fig. 3d; the fluorescence-activated cell sorting (FACS) gating strategy and expression of marker genes in S1, S2 and S3 subsets are shown in Extended Data Fig. 3a–c). As CAFs in the S3 subset, and those in S2 to a lesser degree, expressed higher levels of the pericyte markers Rgs5 and Cspg4 (coding for NG2) than did those in S1 (Extended Data Fig. 3b,c), these data are in line with the increased pericyte coverage observed in tumors depleted of ADAM12+ cells (Fig. 2i). We further investigated the mechanism. The decrease in S1 CAFs was likely not due to direct ablation of ADAM12+ cells, as most ADAM12+ cells had low or no expression of DPP4 and medium or low expression of CD34, consistent with previous scRNA-seq data9 (Extended Data Fig. 3e,f), and represented a small percentage of total stromal cells (Fig. 1c). Because ablation of ADAM12+ cells decreased tumor hypoxia (Fig. 2f), we asked whether hypoxia affected CAF differentiation toward S1. Accordingly, we observed that hypoxia induced upregulation of Cd34, Dpp4, C3, Il6ra and Il6st (marker genes of the S1 stromal population; Extended Data Fig. 3b) in stromal cells in vitro, whereas expression levels of genes coding for broad fibroblast markers, such as Pdgfra and Pdpn, were unaffected (Extended Data Fig. 3g). These data are in line with previous reports showing that inflammatory CAFs are enriched in tumor hypoxic regions45,46 and further suggest that depletion of ADAM12+ MSCs normalizes CAFs by decreasing tumor hypoxia.
a, Immunofluorescence staining of PDPN, CD3 and CD31 in MO5 tumors of ADAM12-DTR mice (DTR) and littermates (Ctrl) treated with DT from day 10. One representative image of several independent experiments (n = 4) is shown. Scale bars, 100 μm. b, Tumor-infiltrating CD3+ T cells in mice treated as in a; n = 7 in 2 independent experiments. c, Percentage of PDPN+PDGFRα+ cells (gated CD45–CD31–), measured by FACS, in MO5 tumors of mice treated as in a; n = 12 (Ctrl)–14 (DTR) in 3 independent experiments. d, Percentage of MO5 tumors containing Pdpn+ stromal cells, as indicated in mice treated as in a. n = 6–8 in 4 independent experiments. e, Volcano plot showing differentially expressed (DE) genes in PDPN+PDGFRα+ cells isolated from DTR versus Ctrl tumors. f, Tumor hypoxia was assessed by immunofluorescence staining of pimonidazole in tumors treated as in a (n = 4). Scale bars, 100 μm. g, Extracellular pH in tumors growing in mice treated as in a, or in Ctrl mice treated with U-104. n = 10(Ctrl)–11(DTR) in 3 independent experiments. h, Expression of Car9, as measured by qRT–PCR, in tumors from mice treated as in a; n = 9 (Ctrl)–10 (DTR) in 2 independent experiments. i, Immunofluorescence staining of NG2+ pericytes and CD31+ blood vessels in tumor sections from mice treated as in a. Left, n = 22 (Ctrl)–32 (DTR) and right, n = 15 (Ctrl)–28 (DTR) blood vessels. j, Immunofluorescence staining of ICAM1, CD45 and CD31 in tumor sections from mice treated as in a; n = 11 (Ctrl)–17 (DTR) fields. k, Tissue perfusion, as detected by Hoechst 33342 staining, in tumor sections from mice treated as in a; n = 10 (Ctrl)–8 (DTR). Scale bar, 200 μm. In i–k, data are representative of several independent experiments (3–5). In i–j, scale bars, 50 μm. Statistics were calculated using two-tailed, unpaired Student’s t-test (f,h,i(right),j,k), ordinary one-way ANOVA (g), two-tailed Mann–Whitney test (b,c,i(left)) or two-sided Wald test (DESeq2) (e). All quantitative data are presented as means ± s.d.
Slow-cycling ADAM12+ MSCs regulate the TME in a TGF-β-dependent way
These data show that a small subset of ADAM12+ MSCs developing early at the tumor margins strongly affect the vascular and stromal tumor microenvironment. To investigate the underlying mechanism, we performed a transcriptome analysis of ADAM12+ cells isolated from MO5 melanomas at day 8. Differential gene expression analysis identified >700 genes that were significantly upregulated in ADAM12+PDPN+PDGFRα+ cells compared with ADAM12–PDPN+PDGFRα+ cells (Fig. 3a). Gene set enrichment analysis indicated that among the top enriched pathways for ADAM12+ cells were terms related to ECM remodeling, cell proliferation and differentiation, and inflammation, including major signaling pathways downstream of growth factors receptors (RTK) and cytokine receptors, such as phosphoinositide 3-kinase (PI3K)–Akt, MAPK, nuclear factor-κB (NF-κB), tumor necrosis factor (TNF) and JAK–STAT signaling pathways (Fig. 3b). Several pathways related to cell metabolism, protein synthesis and DNA replication were downregulated in ADAM12+ cells compared with ADAM12– cells (Fig. 3c). In line tumors with ADAM12+ cells having a lower metabolism, ADAM12+ cells downregulated expression of genes involved in cell proliferation, glycolysis and oxidative phosphorylation, such as Mcm2, Cdk2, Cdk15, Ldhb, Ldha and Atp5a1, and upregulated expression of Gadd45b, Gadd45g and Gpx3, which are induced upon cell cycle arrest in response to oxidative or stress damage (Fig. 3d). Further suggesting that there is crosstalk within the TME, ADAM12+ cells expressed higher levels of Pdgfra, Il1r1 (which encodes an activator of NF-κB), Osmr and Il6st (gp130), which encodes a signal transducer in a receptor complex involving several cytokines, including oncostatin (OSM) and interleukin-6 (IL-6) (Fig. 3d). They also upregulated Il6, genes encoding chemokines including Cxcl1, and genes with key roles in monocyte recruitment and macrophage polarization, such as Ccl2, Ccl7, Csf1 and Has2 (Fig. 3d), as well as growth arrest specific 6 (Gas6), Lgals3 and Pros1, which promote macrophage efferocytosis through the TAM (TYRO3, AXL and MERTK) tyrosine kinase receptors47. In addition, ADAM12+ cells upregulated Icam1, which encodes an adhesion molecule, and protumor components of the ECM such as Ctgf, Lox, Hspg2, Mmp3 and Tgfbr3 (Fig. 3d)48,49,50, suggesting that these cells have a role in TME remodeling. Compared with ADAM12–PDGFRα+ cells, ADAM12+PDGFRα+ cells upregulated genes overexpressed by mesenchymal progenitors, such as Ngfr, Ly6a (sca-1), Vcam1 (CD106) and PDGFRβ, but not Acta2 (coding for αSMA) or Lrrc15 (Fig. 3d and Extended Data Figs. 1g and 4a). These data suggest that ADAM12+ cells are perivascular mesenchymal progenitors that are distinct from αSMA+ myofibroblasts and Lrrc15+ CAFs51.
a, Heat map of RNA-seq differential gene expression analysis of ADAM12+ PDPN+PDGFRα+ (GFP+) versus ADAM12–PDPN+PDGFRα+ (GFP–) CD45–CD31– cells isolated by FACS from MO5 tumors growing in ADAM12-GFP mice (n = 4). b,c, Pathway enrichment analysis of genes significantly upregulated (b) or downregulated (c) in GFP+ cells, using KEGG annotation. d, Differential gene expression analysis (GFP+ cells versus GFP– cells). The bar plots represent log2(fold change). e, Left, immunofluorescence staining of the indicated markers in MO5 tumors growing in ADAM12-GFP mice. One representative image of three to four independent experiments is shown. Right, quantification of staining in the left panel. Scale bars, 50µm. f, Growth curve of GFP+ and GFP– cells isolated from MO5 tumors, n = 4 (GFP+)–7 (GFP–). OD, optical density. g, Expression of the indicated transcripts, measured by qRT–PCR, in ADAM12– PDGFRα+ cells isolated from MO5 tumors and treated with TGF-β; n = 3 (–TGF-β)–4 (+TGF-β), except for Adam12, n = 5 (–TGF-β)–4 (+TGF-β). h, Expression of the indicated transcripts, measured by qRT–PCR, in ADAM12+PDGFRα+ cells treated with IL-1β or OSM (fold change treated versus non treated). +IL-1β, n = 3, except for Il6, n = 5; OSM, n = 4, except for Mmp3 and Gadd45g, n = 3. Statistics were calculated using one-way ANOVA (e), two-way ANOVA (f) or two-tailed, unpaired Student’s t-test (g,h). All quantitative data are presented as means ± s.d.
The majority of ADAM12+ MSCs did not express Ki-67 or incorporate Edu, in contrast to CAF populations (Fig. 3e and Extended Data Fig. 4b), consistent with the cells being in a slow-cycling state. The absence of senescence markers, such as Cdkn2a (p16-INK4A) and Cdkn1a (P21), argued against a senescent state, characterized by an irreversible arrest of cell proliferation and growth. Accordingly, and differently from senescent cells, which are unresponsive to mitogenic stimuli, ADAM12+ MSCs rapidly resumed cell proliferation when given nutrients in vitro (Fig. 3f). TGF-β has a major role in cell cycle regulation52. In addition to inducing Adam12 expression (consistent with previous reports24,26,27), TGF-β rapidly induced expression of Gadd45b and Gadd45g, which encode cycle-arrest proteins, in ADAM12–PDGFRα+PDPN+ cells isolated from MO5 tumors, and downregulated cyclins involved in cell proliferation, such the one encoded by Cdk6 (Fig. 3g). ADAM12+ cells overexpressed a number of genes encoding receptors for inflammatory cytokines and growth factors (Fig. 3d), including Il1r1, Osmr and Il6st, suggesting that there was additional crosstalk within the TME. Within the TME, TAMs expressed the highest levels of Il1b, Osm and Pdgfc (Extended Data Fig. 4c). Although IL-1β and OSM did not induce Adam12 expression (Extended Data Fig. 4d), IL-1β induced Cxcl1, Ccl2, Il6 and Mmp3 expression in ADAM12+ MSCs isolated from MO5 tumors, and OSM rapidly induced expression of Il6, Icam1, Has2, Il1r1 and Gadd45g (Fig. 3h). Additional factors overexpressed by ADAM12+ cells, such as Gas6, were strongly induced by starvation in stromal cells (Extended Data Fig. 4e), overall suggesting that the inflammatory and nutrient-deprived TME further modulated the phenotype of TGF-β-induced ADAM12+ cells.
Tgfb1 was upregulated by several cell types within the TME in early tumor stages, and TAMs were major producers of Tgfb1 in advanced tumors (Extended Data Fig. 4f,g). As ADAM12+ cells expressed high levels of Tgfbr2 (Extended Data Fig. 4h,i), and ADAM12 enhanced TGF-β signaling in vitro27, we investigated the role of TGFBR2 in ADAM12+ cells in MO5 tumors in vivo. To that aim, we generated a tetracycline-regulated ablation model of Tgfbr2 in ADAM12+ cells by crossing ADAM12-tTA mice27 with tet-controlled Cre (LC-1 mice) and Tgfbr2loxP/loxP mice (ADAM12-tTA-CreTgfbr2 mice, Extended Data Fig. 4j). As TGF-β expression is induced at early tumor stages (Extended Data Fig. 4f), we started ablating Tgfbr2 in ADAM12+ cells by removing doxycycline at the initiation of tumorigenesis (Extended Data Fig. 4k). We observed significant inhibition of tumor growth, as well as increased infiltration of T cells, in ADAM12-tTA-CreTgfbr2 mice compared with WT littermate mice (Extended Data Fig. 4l,m), showing that TGFBR2 is required for the pro-tumorigenic role of ADAM12+ cells. Macrophages isolated from MO5 tumors growing in ADAM12-tTA-CreTgfbr2 mice expressed lower levels of Vegfa, a major inducer of leaky vessels, and tumor vessels had improved pericyte coverage (Extended Data Fig. 4n,o), consistent with blood vessel normalization. Accordingly, stromal cells isolated from MO5 tumors growing in ADAM12-tTA-CreTgfbr2 mice expressed higher levels of Angpt1 and Pdgfrb, which stabilize the vasculature through pericyte–endothelial cell interaction53, and lower levels of Car9, induced in hypoxia39, compared with stromal cells isolated from WT tumors (Extended Data Fig. 4p). These data show that TGFBR2 signaling in ADAM12+ cells is required for their pro-tumorigenic function and TME alterations.
ADAM12+ MSCs promote macrophage efferocytosis and polarization
Further suggesting a crosstalk between ADAM12+ MSCs and macrophages, ADAM12+ cells accumulated around MO5 tumors close to CD206+ TAMs, starting in early tumor stages (Fig. 4a). In larger tumors, ADAM12+ cells remained localized at the tumor margin, and a majority (>80%) were in close proximity to macrophages with phosphorylated AXL (AXL-P+, Fig. 4b), a receptor that is essential for efferocytosis, and were distant from T cells (Extended Data Fig. 5a). By contrast, only 20–40% of CAFs were adjacent to AXL-P+ macrophages (Extended Data Fig. 5b). Engulfment of apoptotic cells by macrophages through efferocytosis induces anti-inflammatory cytokines such as TGF-β, IL-10 and prostaglandin E2 (PGE2), which promote tumorigenesis54,55. Efferocytosis is enhanced by molecules such as Gas6, which bridges phospatidylserine on apoptotic cells with TAM receptors54. Of note, Gas6 has a higher affinity for AXL receptors than for Mertk or Tyro3 receptors, which were expressed at lower levels on tumor macrophages (Fig. 4c). In line with our sequencing data, PDPN+PDGFRα+ cells had a higher Gas6 expression level than did other cell types in the tumors (Extended Data Fig. 5c); ADAM12+PDPN+PDGFRα+ cells had particularly high Gas6 expression levels, both at the RNA and protein level (Fig. 4d,e). Although CAFs did not show efferocytic activity (Extended Data Fig. 5d), the conditioned medium (CM) from ADAM12+ MSCs (GFP+ cells) isolated from MO5 tumors increased efferocytosis of bone-marrow-derived macrophages (BMDMs), an effect that was inhibited when BMDMs lacked AXL in myeloid cells (isolated from LysM-CreAXLfl/fl mice) (Fig. 4f, AXLfl/fl littermates were used as WT controls). Efferocytic BMDMs treated with CM from GFP+ cells expressed higher levels of Tgfb1, Vegfa and Il10 than did BMDMs treated with CM from GFP– cells (Extended Data Fig. 5e), consistent with efferocytosis-induced immunosuppression54,56. A similar process occurred in vivo: depletion of ADAM12+ cells induced a significant decrease of AXL-P+ macrophages (Fig. 4g), whereas the frequencies of apoptotic cells, mostly non-perivascular and non-stromal cells, were increased (Fig. 4h and Extended Data Fig. 5f). Accordingly, the engulfing ability of macrophages isolated from tumors lacking ADAM12+ cells was decreased compared with that of macrophages isolated from WT tumors (Extended Data Fig. 5g). These data are consistent with decreased expression of Gas6 in tumors depleted of ADAM12+ cells compared with WT tumors (Extended Data Fig. 5h), showing that ADAM12+ cells are a significant source of Gas6 within the TME. In line with a role for ADAM12+ cells in this process in vivo, the ratio of major histocompatibility complex II (MHCII)hiCD206lo inflammatory macrophages to MHCIIloCD206hi TAMs significantly increased in tumors lacking ADAM12+ MSCs (Fig. 4i). Macrophages isolated from tumors lacking ADAM12+ cells expressed lower levels of Tgfb1, Il10, Ptgs2 (coding for PGE2) and Vegfa (Fig. 4j) and upregulated Light (Tnfsf14), which promotes antitumor immunity by activating NK cells, T cells, stromal cells and restoring blood vessels integrity57,58. Accordingly, treatment with anti-AXL activating antibodies59 restored tumor progression in MO5 tumors lacking ADAM12+ cells, and increased the AXL-P+ macrophage level to that in WT mice (Fig. 4k,l), whereas it had no effect on WT tumors. Overall, these data show that ADAM12+ MSCs are induced at early tumor stages and polarize tumor macrophages toward an immunosuppressive and proangiogenic phenotype by promoting efferocytosis.
a,b, Immunofluorescence staining of the indicated markers in MO5 tumors at 8 d (a) and 12 d (b) growing in ADAM12-GFP mice. Scale bar in a, 100 µm (left, middle) and 50 µm (right); in b, 50 µm. In b, distance of GFP+ cells to AXL-P+ macrophages (right). Results are representative of four independent experiments. c, Gene expression of Axl, Mertk and Tyro3, measured by qRT–PCR, in macrophages isolated from MO5 tumors (n = 4). d, Gene expression of Gas6, measured by qRT–PCR, in ADAM12+ and ADAM12– cells isolated from MO5 tumors; n = 4 from independent experiments. e, Secretion of Gas6, measured by ELISA (n = 3). Results are representative of three independent experiments. f, Efferocytosis test in BMDMs isolated from the indicated mice in presence of CM obtained from GFP+ or GFP– cells. n = 9 (+CM GFP+/GFP–) and n = 6 (Ctrl medium). Results are representative of three independent experiments. g,h, Left, immunofluorescence staining of the indicated markers in tumors growing in DTR or Ctrl mice injected with DT. Right, quantification of expression, which was performed on n = 12(Ctrl)–11(DTR) in g and n = 9(Ctrl)–12(DTR) in h. Results are representative of three independent experiments.White arrowheads indicate F480+AXL-P+ macrophages (g) and cleaved-C3+ apoptotic cells (h). Scale bars, 50 μm. i, FACS plot and percentages of macrophages expressing MHCII and CD206 in tumors growing in DTR or Ctrl mice treated with DT (left). Ratio of MCHIIhiCD206lo (M1) over MCHIIloCD206hi (M2) macrophages (right). n = 12(Ctrl)–11(DTR) from 2 independent experiments. j, Expression of the indicated transcripts, measured by qRT–PCR, in macrophages isolated by FACS from tumors growing in mice treated as in i; Vegfa, n = 13(Ctrl)–12(DTR); Tgfb1, n = 13(Ctrl)–10(DTR); Light, n = 9(Ctrl)–10(DTR); Il10, n = 14(Ctrl)–13(DTR); Ptgs2, n = 11(Ctrl)–9(DTR). Results are representative of three independent experiments. k, Tumor growth curves of DTR and Ctrl mice treated with DT and activating anti-AXL antibodies or isotype control (IgG). Left, average tumor volume; right, growth curves for individual animals; n = 4, except for Ctrl + IgG (n = 6). The x axis represents days after tumor inoculation. l, Percentage of AXL-P+ macrophages in tumor sections from mice treated as in k. Quantifications were performed on n = 10 (Ctrl + IgG), n = 4 (Ctrl + α-AXL), n = 6 (DTR + IgG), n = 8 (DTR + α-AXL) fields. Statistics were calculated using one-way ANOVA (b), two-tailed, unpaired Student’s t-test (d,e,g,i,j), Mann–Whitney U test (h,j Ptgs2) or two-way ANOVA (f,k,l). All quantitative data are presented as means ± s.d.
Tumor-induced ADAM12+ lineage is maintained in advanced stages
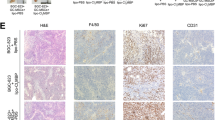
To determine whether ADAM12+ MSCs develop in spontaneously occurring tumors, we crossed ADAM12-GFP mice with Rip-Tag2 mice, a mouse model of neuroendocrine pancreatic tumor, or with TRAMP mice, a mouse model of prostate adenocarcinoma. In these models, expression of the SV40 large T antigen drives multi-step tumor progression with tumor stages similar to those in human cancer. In both tumors, we observed PDPN+ stromal cells and macrophages accumulating in peritumoral areas (Extended Data Fig. 6a,b). We observed around 1–6% of stromal cells expressing ADAM12 and localized close to blood vessels at the margins of Rip-Tag2 and TRAMP tumors, but not in normal pancreas or prostate (Fig. 5a,b). Similar to what happened in the melanoma, ADAM12+ cells, in comparison to ADAM12– cells, upregulated genes involved in cell cycle arrest, growth factors and cytokine receptors, as well as genes regulating macrophages and the ECM, including Gas6, Csf1, Has2, Mmp3 and the mesenchymal progenitor markers Ly6a and Vcam1, and downregulated Mki67, Cdk1, Cdkn2a and Acta2 (Extended Data Table 1 and Extended Data Fig. 7a). ADAM12+ cells expressed low to medium levels of PDGFRβ and were localized outside the ColIV+ vascular BM (Fig. 5a, left, Extended Data Fig. 7a, right). To determine the fate of ADAM12+ MSCs in vivo during tumor progression, we generated a tetracycline-regulated lineage-tracing system of ADAM12+ cells by crossing ADAM12-tTA mice27 with LC-1 mice and Rosa26STOPfloxYFP reporter mice (ADAM12-tTA-CreYFP mice, Fig. 5c). After inoculating MO5 tumor cells in ADAM12-tTA-CreYFP mice (maintained on doxycycline until tumor injection), we observed that yellow fluorescent protein (YFP)+ cells (progeny of ADAM12+ cells) constituted about 1% of stromal cells in advanced melanomas. YFP+ cells were localized at the tumor margins, close to vessels and within the stroma, and expressed varying levels of NG2 and αSMA (Extended Data Fig. 7b). Compared with ADAM12+ cells, YFP+ cells downregulated several factors that are essential for immune-cell crosstalk, including Ccl2, Csf1, Gas6, Lgals3, Cxcl12, Il1r1, Osmr, Il6 and Angpt1, while upregulating varying levels of Car9, Angpt2, Acta2 and Pdgfrb (Extended Data Fig. 7c). As Car9 and Angpt2 encode proteins that block antitumor immunity40,41,60, these data suggest that both ADAM12+ cells and their progeny promote immunosuppression, although through different mechanisms. To determine whether a similar lineage develops in tumors that arise spontaneously, we crossed ADAM12-tTA-CreYFP mice (Fig. 5c) with TRAMP mice or Rip-Tag2 mice. In prostate and pancreatic tumors, we observed YFP+ cells within the stroma and close to blood vessels that expressed medium to low levels of αSMA and NG2 (Fig. 5e and Extended Data Fig. 7d). Although they were in proximity to blood vessels, YFP+ cells were localized outside the vascular BM, in contrast to pericytes61, consistent with detached pericyte-like cells or perivascular αSMAmid fibroblastic cells (Extended Data Fig. 7d). By removing doxycycline at different stages of tumorigenesis (Fig. 5d), we observed that ADAM12+ cells induced at early tumor stages in prostate and pancreatic tumors had increased stromal progenitor potential (Fig. 5f, early, and Extended Data Fig. 7e), compared with ADAM12+ cells at later tumor stages (Fig. 5f, late, and Extended Data Fig. 7e). After treating with doxycycline 10-week-old TRAMP x ADAM12-tTA-CreYFP mice that had been fate mapped from week 4 (Fig. 5g), we analyzed the fate of ADAM12+ cells induced specifically at early tumor stages. In this setting, we observed YFP+αSMA– or YFP+αSMAmid cells at the tumor margins of large prostate adenocarcinomas in 30-week-old TRAMP mice, demonstrating that ADAM12+ cells induced in early tumors generate a peritumoral lineage that is maintained during tumor progression (Fig. 5h). In line with the gene expression data (Extended Data Table 1), YFP+ cells were mostly negative for the proliferation marker Ki-67 (Fig. 5e, right panel). A slow-cycling YFP+Ki-67– cells were also abundant at the interface of normal tissue and SV40+ prostate cancer cells that metastasized to the liver and bone in TRAMP × ADAM12-tTA-CreYFP mice (Fig. 5i), further suggesting a role for ADAM12+ MSCs in the metastatic niche. To investigate the developmental origin of tumor-induced ADAM12+ MSCs, we performed lineage tracing of ADAM12+ cells from development, because ADAM12 is expressed during organ morphogenesis27. In ADAM12-CreYFP mice, we observed that a majority of stromal cells in healthy skin, prostate and pancreas in adults were generated from fetal ADAM12+ progenitors (Extended Data Fig. 7f), suggesting that tumorigenesis reactivates a developmental program. Finally, to assess the role of ADAM12+ MSCs in a spontaneous tumor model, we crossed ADAM12-DTR mice with Rip-Tag2 mice to generate RIP+DTR+ and RIP+DTR– mice. Depletion of ADAM12+ cells induced significant growth inhibition of RIP tumors, which displayed increased infiltration of CD3+ T cells (Extended Data Fig. 7g–i). As observed in the melanoma, PDPN+PDGFRα+ CAFs were reorganized but still present in similar numbers in tumors lacking ADAM12+ MSCs, and the vasculature displayed increased pericyte coverage and ICAM1 expression. Tumors were better perfused (Extended Data Fig. 7j–n), overall confirming a similar role for ADAM12+ cells in this tumor model. These data show that slow-cycling ADAM12+PDGFRα+αSMA– perivascular MSCs induced at early stages of tumorigenesis generate a discrete mesenchymal lineage that is maintained and active in advanced carcinomas and metastasis.
a, Immunofluorescence staining of ADAM12+ cells (GFP), co-stained with the indicated markers, in Rip-Tag2 (left) and TRAMP (right) tumors growing in ADAM12-GFP mice. Scale bars, 50 µm. b, Percentage of GFP+ cells among the indicated populations in TRAMP tumors (n = 3), RIPTag tumors (n = 4 for stroma, n = 3 for CD45+ and CD31+ cells), Ctrl prostate (n = 3) and Ctrl pancreas (n = 2), measured by FACS. Results are representative of two independent experiments. c, Strategy for inducible fate mapping of ADAM12+ cells. tTA, tetracycline transactivator; Dtr, diphteria toxin receptor; Ires, internal ribosomal entry site; Luc, luciferase; TRE, tet-responsive element; hCMV, human cytomegalovirus; Dox, doxycycline, LC-1, Luciferase_Cre transgenic mice74. d, Experimental setup for lineage tracing of ADAM12+ cells induced de novo at early or late tumor stages in TRAMP or Rip-Tag2 mice. e, Immunofluorescence staining of YFP and the indicated markers, in Rip-Tag2 and TRAMP tumors growing in ADAM12-tTA-CreYFP mice. Scale bars, 50 μm. f, Percentage of YFP+ cells among total tumor stromal cells, measured by FACS, from mice treated as in d. n = 4 (RIP early), n = 6 (RIP late), n = 4 (TRAMP early), n = 2 (TRAMP late), n = 2 (Ctrl). g, Experimental setup for lineage tracing of ADAM12+ cells induced at early stages of tumorigenesis. h, Immunofluorescence staining of the indicated markers in TRAMP prostate tumors growing in ADAM12-tTA-CreYFP mice treated as in g. Scale bar, 100 µm. i, Immunofluorescence staining of the indicated markers in TRAMP tumors that metastasized in the liver (left and middle) or bone (right) in ADAM12-tTA-CreYFP mice. SV40 stains tumor cells. Scale bars, 50 µm. In a, e, h, and i, images are representative of independent experiments (n = 3–6). Quantitative data are presented as means ± s.d. Met, metastasis. DAPI stains nuclei.
ADAM12 can stratify patients with cancer across tumor types
ADAM12 is overexpressed in several solid tumors, including melanoma, prostate, breast, liver, colorectal and pancreatic tumors22,23,24,25,26 and is associated with stromal activation and a poor prognosis23,26,28,29,30,31,32,33. As observed in mice models, ADAM12 was preferentially expressed by stromal populations in several human tumors (Extended Data Fig. 8a–e), consistent with previous reports24,62,63,64. In human pancreatic ductal adenocarcinoma, ADAM12 expression was correlated with the ‘stroma activated’ subtype, which is associated with a severe prognosis65 (Extended Data Fig. 8d). We further analyzed ADAM12 expression in publicly available datasets from cohorts of patients with skin cutaneous melanoma, pancreatic ductal adenocarcinoma, prostate adenocarcinoma or colon adenocarcinoma. For each cohort, we stratified tumors by ADAM12 expression (ADAM12 expression < median expression versus ADAM12 expression > median expression). Gene Set Enrichment Analysis of pathways using the Hallmark gene sets indicated that, independently of the tumor type, ADAM12hi tumors were significantly enriched in genes related to the terms ‘Inflammatory response’, ‘Hypoxia’ and ‘Angiogenesis’, and were inversely correlated with ‘Oxidative phosphorylation’ (Fig. 6a), a process that requires oxygen. Pathway analysis using the Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) database in these tumors further indicated enrichment for the terms ‘Cytokine–cytokine receptor interaction,’ ‘ECM–receptor interaction,’ ‘Tissue remodeling’ and ‘Regulation of wound healing’ (Fig. 6a,b). Analysis of the leading edge (i.e., genes accounting for a pathway being defined as enriched) in the pathways ‘Cytokine–cytokine receptor interaction’, ‘ECM–receptor interaction’, ‘Inflammatory response’ and ‘Tissue remodeling’ further identified a number of shared genes in the four tumor datasets (Fig. 6b–e), suggesting that there is a shared gene expression program across solid tumors grouped by ADAM12 expression. Notably, and similar to the data obtained in mice models, common genes of the leading edge in the four human datasets included genes regulating or expressed by tumor macrophages, such as AXL, CSF1, CSF1R, CD14 and MSR1, and genes encoding cytokines and cytokine receptors of the OSM, IL-6 and PDGF pathways, as well as genes encoding structural and regulatory proteins of the ECM with essential roles in tissue remodeling and angiogenesis, including collagens, laminins, HSPG2, HAS2, KDR and NOX4. Consistent with increased resistance mechanisms, stratification of human prostate cancer by ADAM12 expression further correlated with the Gleason score, which identifies high-grade tumors at high risk of recurrence (Extended Data Fig. 8f). These data show that, in several desmoplastic tumors, including pancreatic, prostate and colon cancer, ADAM12 expression stratifies patients harboring tumors with high levels of hypoxia, inflammation, tissue remodeling and innate resistance mechanisms, as well as factors associated with a poor prognosis and drug resistance such as AXL.
a, GSEA of pathways in ADAM12hi versus ADAM12lo tumors (median expression) of human pancreatic ductal adenocarcinoma (ICGC_PAN_AU; yellow, n = 267), colon carcinoma (TCGA_COAD; green, n = 450), melanoma (TCGA_SKCM; navy, n = 147) and prostate adenocarcinoma (TCGA_PRAD; gray, n = 455) datasets. The bar plots represent the –log10(P value) and are colored according to the normalized enrichment score (NES) (red, positive; blue, negative; the value is shown at the end of each bar plot). b–e, Gene set enrichment analysis (GSEA) curves for the indicated pathways for the four tumor datasets analyzed as in a (right, red square indicates the leading-edge genes). For each pathway, to the left of the GSEA curves, the heat map shows the expression levels of the genes present in the leading edge and shared between the four datasets. All patients whose data have been analyzed are represented, and whether they have high (green) or low (red) ADAM12 expression is indicated above the heat map. A Wilcoxon rank-sum test was used for pre-rank GSEA for statistical analysis.
Discussion
Here we show that tumor-induced stromal cell cycle arrest at the tumor margin coordinates angiogenesis, tissue remodeling and immunosuppression, key drivers of tumor progression. Selective depletion of such metabolically altered mesenchymal stromal subset, identified by expression of ADAM12, normalized the TME and decreased tumor hypoxia and acidosis, inducing infiltration of activated T cells and inhibition of tumor growth. We further provided direct genetic evidence that TGFBR2 signaling in ADAM12+ MSCs is required for their pro-tumorigenic function.
We showed that ADAM12+ MSCs are induced by TGF-β, a major cytostatic factor that is upregulated in early stages of tumorigenesis5, and are further modulated by IL-1β and OSM, which have pivotal roles in cancer-associated inflammation and tumor initiation66. These results are consistent with previous reports showing that the NF-kB and PI3K pathways, activated by IL-1β and OSM, respectively, are associated with alterations of tumor-associated stroma cells and TAMs, vascularization and ECM remodeling67,68,69. Specifically localized in the perivascular niche at the tumor margins, ADAM12+ cells were major producers of factors regulating monocyte recruitment and macrophage function and polarization, including Ccl2, Csf1 and Has2, as well as bridging molecules enhancing macrophage efferocytosis, such as Gas6, and promoted macrophage efferocytosis in an AXL-dependent way. Efferocytosis is a fundamental mechanism in prevention or resolution of tissue inflammation. In the context of cancer, it favors immunosuppression, angiogenesis and tumor progression54,55,56. Consistent with inhibition of macrophage polarization toward proangiogenic TAMs, depletion of ADAM12+ MSCs induced normalization of the tumor vasculature. ADAM12+ MSCs are restricted to the tumor margin, further suggesting that they spatially coordinate with TAMs to relay signals from the margin.
We show that, in tumors lacking ADAM12+ MSCs, the total number of CAFs was not reduced, but rather these cells became immunopermissive. We did not detect any significant differences in transcripts coding for collagens or chemokines in immunopermissive CAFs, consistent with previous reports showing that structural, vascular and immune homeostatic functions of CAFs are essential to restrain tumor growth15,16,17,18,19,20,21. The major transcriptomic changes in immunopermissive CAFs were downregulation of hypoxia-induced genes such as Car9 (CAIX), which promotes tumor acidification, a strong immunosuppressive factor. Consistent with restoration of normoxia, depletion of ADAM12+ MSCs increased the coverage of blood vessels with NG2+ pericytes, essential for vascular stabilization and function, and the number of inflammatory CAFs was reduced. These data are consistent with previous reports showing that inflammatory CAFs are enriched in tumor hypoxic regions45,46, and further suggest that hypoxia is a major driver of immunosuppressive functions in CAFs35,36,37,38,39.
ADAM12+ MSCs were in a ‘dormant’ slow-cycling state, distinct from senescence, as they were able to resume the cell cycle when provided with nutrients. The capacity to enter a slow-cycling state is a common feature of stem-like cells that promotes resistance to limited resources and antimitotic therapies, evasion from the immune system and dissemination of tumor cells6. Our data are consistent with a protumor effect of cytostasis, mediated by TGF-β, on perivascular MSCs, as selective depletion of ADAM12+ MSCs or conditional ablation of Tgfbr2 in ADAM12+ MSCs was sufficient to inhibit tumor growth. Therefore, TGF-β might promote pro-tumorigenic functions through different mechanisms in MSCs and CAFs51. Albeit distinct from pericytes, ADAM12+ MSCs and their progeny remained within the perivascular niches of the TME for months, resembling αSMAmid fibroblastic cells or detached pericyte-like cells. These populations have major roles in TME alterations and immunosuppression, notably by affecting perivascular TAMs and vascular function42,70. Finally, these data suggest that antitumor therapies aimed at promoting cell cycle arrest, cell damage or starvation might promote immunosuppression by inducing a low proliferative stem-like state in tumor-associated MSCs.
ADAM12 has been identified as a marker for stromal activation, poor prognosis and resistance to therapy in several human desmoplastic tumors, including pancreatic, liver, colorectal, lung, breast and ovarian cancers23,26,28,29,30,31,32,33. In agreement with a role for ADAM12 in this process, knock out of Adam12 in a murine model of prostate cancer blocked tumor progression to poorly differentiated advanced stages22. We further show that ADAM12 expression stratifies patients with high levels of inflammation, hypoxia, innate resistance mechanisms, and tissue remodeling. These results are in line with increasing evidence that hypoxia and acidosis suppress antitumor immune responses4,71,72,73. Altogether, these data suggest that ADAM12+ MSCs are part of a fundamental repair process that is initiated by cytostatic TGF-β and modulated by inflammation, to promote angiogenesis and anti-inflammatory responses in coordination with macrophages. We propose that the pathological persistence of the ADAM12 lineage in tumors, which is normally eliminated from the tissue when healing is complete27, plays a key role in inducing and maintaining an immunosuppressive and hypoxic TME. Although hypoxia and acidosis promote tumor malignancy, they are essential components of the repair process, notably for pathogen clearance and restoration of tissue homeostasis, suggesting an evolutionary advantage.
Methods
Mice
We have previously described the generation of BACs (bacterial artificial chromosomes) transgenic mice ADAM12-GFP (Cre-Ires-GFP) and ADAM12-DTR (tTA-Ires-DTR)27. To perform inducible lineage tracing of ADAM12+ cells, we crossed ADAM12-tTA mice with tetracycline-controlled Cre (LC-1 mice74) and Rosa26floxSTOP-YFP reporter mice (obtained from The Jackson Laboratory) to obtain ADAM12-tTA-CreYFP triple transgenic mice. TGFBR2flox mice75 were obtained from The Jackson Laboratory. All mice were housed in specific-pathogen-free conditions (14 h light/10 h dark, 20–24 °C, 50% humidity). Mice experiments were approved by the committee on animal experimentation of the Institut Pasteur and by the ‘Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche.’
Tumor models
The B16-OVA (MO5) melanoma cell line (B16 cell line containing the ovalbumin gene76) was provided by C. Leclerc (Institut Pasteur). For melanoma studies, 5 × 105 MO5 cells were implanted subcutaneously in 8–12-week-old transgenic mice and sex-matched non-transgenic littermates, in the C57Bl/6 background, and tumor volume was assessed every 2–4 d. Caliper measurements were used to assess tumor volumes, using the formula (L × W2) / 2 (L, length; W, width). Tumors did not exceed the maximum size permitted by the animal experimentation committee (20 mm). In TRAMP mice, the rat promoter Probasin drives expression of SV40 large T antigen, leading to progressive forms of prostate cancer, from intraepithelial hyperplasia to carcinoma with distant-site metastasis77. In Rip-Tag2 mice, the rat Insulin promoter drives expression of SV40 large T antigen, leading to pancreatic β-cell tumors78.
Mice treatment
Depletion and lineage tracing of ADAM12+ cells was performed as we previously described27. Briefly, we injected ADAM12-DTR mice intraperitoneally (i.p.) with 100 ng of diptheria toxin (DT) every day for the indicated time to deplete ADAM12+ cells. To stop lineage tracing of ADAM12+ cells, we treated ADAM12-tTA-CreYFP mice with doxycycline (Sigma-Aldrich) at 1 mg ml−1 in drinking water containing 5% sucrose. The CAIX inhibitor U-104 (38 mg kg–1, Selleck) was injected i.p. every 2 d starting at day 10 following MO5 inoculation. To assess proliferation, tumor-bearing mice were injected i.p. with 80 µl of 10 mM EdU and analyzed 24 h after injection. To inhibit CD8+ T cells, mice were injected i.p. at day 6 and 10 after tumor inoculation with anti-CD8 antibodies (200 µg per mouse, Biolegend no. 100746) or control IgG (Biolegend no. 400544). For analysis of tumor perfusion, the fluorescent stain Hoechst 33342 (H33342) was injected intravenously (i.v.) 1 min before euthanization. To activate AXL, mice were injected i.p. at day 9 and 13 after tumor inoculation with anti-AXL activating antibodies (1 mg kg–1, AF854; R&D Systems) or control IgG (AB-108-C; R&D Systems), as previously described59,79.
Immunofluorescence
Tissues were processed and stained as previously described27. Briefly, 8-µm sections from OCT-frozen tumors were incubated in 10% bovine serum (BS) in PBS containing 0.1% Triton X-100 (PBS-TS), followed by incubation with primary antibodies in PBS containing 1% PBS-TS overnight at 4 °C, washed and incubated for 1 h at 20 °C with secondary antibodies or streptavidin, washed, incubated with DAPI (1 µg ml–1) and mounted with Fluoromount-G (Southern Biotechnology Associates). Expression of Adam12 on tumor sections was detected with an RNAscope ISH assay (Advanced Cell Diagnostics), following the manufacturer’s instructions. We examined slides with an AxioImager M1 fluorescence microscope (Zeiss) equipped with a CCD camera and processed images with AxioVision Zen software (Zeiss) or ImageJ software.
Antibodies
A full list of antibodies used in this study is provided in Supplementary Table 1. We used the following dilutions: anti-GFP polyclonal (rabbit 1:1,000, chicken 1:1,500), anti-CD11b (1:400), anti-MHCII (1:400), anti-NK1.1 (1:200), anti-Foxp3 (1:200), anti-collagen-I polyclonal (1:1,000), anti-collagen-IV (1:1,000), anti-αSMA (1:500), anti-NG2 (1:200), anti-CD31 (1:200), anti-CD3 (1:200), anti-CD4 (1:200), anti-CD45.2 (1:200), anti-Ly6C (1:200), anti-SiglecF (1:500), anti-PDGFRα (1:100), anti-PDGFRβ (1:100), anti-CD8b (1:200), anti-F4/80 (1:200), anti-IFN-γ (1:200), isotype control IgG1 (1:200), anti-ICAM1 (1:400), anti-Ly6G (1:400), anti-CD11C (1:200), anti-CD206 (1:400), streptavidins (1:500), anti-PDPN (1:400), secondary antibodies (1:500), anti-AXL (1:1,000), goat IgG control (1:1,000), anti-cleaved-Caspase3 (1:1,000), anti-P-AXL (1:50), anti-CD26 (1:400), anti-FDC (1:200), anti-MadCAM-1 (1:200), anti-CD34 (1:50), and anti-Ki-67 (1:200).
Cells isolation and FACS
Tumors were cut into small pieces and processed in a solution composed of DMEM (Gibco), Liberase TL (0.26 Wunsch units mL–1; Roche) and DNase I (1 U mL–1; Thermo Fisher) for 30 min, with manual dissociation by pipetting every 10 min. Cells were filtered through a 100-µm and a 40-µm mesh, washed and processed for cell staining as previously described27. Briefly, we first incubated cells with monoclonal antibody 2.4G2 to block Fcγ receptors, and then with the indicated antibodies in PBS containing 2% bovine serum (PBS-BS), followed by appropriate secondary antibodies or streptavidin when necessary. Cells were incubated with DAPI (Sigma) before analysis to exclude dead cells. For FACS analysis of immune cells, tumors were analyzed at day 14–15 except for Treg cells (days 17–18). For intracellular IFN-γ staining, cells were stimulated in vitro for 4 h with PMA and ionomycin, or with SIINFEKL peptide (OVA), and for 2 h with Brefeldin A. Cells were incubated with the LIVE/DEAD Cell Stain Kit to exclude dead cells (Invitrogen). Cells were processed for intracellular staining using CytoFix/CytoPerm Buffer Kit (Invitrogen), according to the manufacturer’s instructions. Cells were analyzed with Fortessa (BD Biosciences) and Flowjo software (Tristar) and sorted with FACS ARIA III (BD Biosciences). For cell sorting of stromal cells, dead cells d doublet cells, hematopoietic (CD45+) and endothelial (CD31+) cells were systematically gated out before selecting for positive stromal cell markers. Tumor macrophages were sorted as CD45+CD3–CD19–Ly6G–SiglecF–CD11b+F480+. Stromal cells of the LN were gated as CD45–CD31– PDPN+ (FRC), which were further selected for expression of MAdCAM-1 (MRC) or FDC-M1 (FDC). TRC were defined as CD45–CD31– PDPN+MAdCAM-1–FDC-M1–.
Efferocytosis assay
To induce apoptosis, MO5 melanoma cells were serum starved for 2 h, irradiated with UV (UVP Crosslinker at 100 mJ cm–2) for 10 min and incubated overnight in complete medium. Apoptotic MO5 cells were incubated with Amine-Reactive pHrodo Dyes (Thermo Fisher) at a concentration of 20 ng mL–1 for 30 min at 20 °C in the dark. For the efferocytosis assay, BMDMs were mixed with pHrodo-stained apoptotic MO5 cells for 30 min at 37 °C, at a 1:1 ratio, in the presence of conditioned medium from GFP+ or GFP– cells, stained with antibodies anti-F4/80 and analyzed with Fortessa (BD Biosciences) and Flowjo software (Tristar). The fold changes on the y axis were calculated using the percentage of efferocytosis in control medium as the baseline.
RNA Isolation and qRT–PCR
For total tissue RNA extraction, we used the PureLink RNA Mini Kit (Invitrogen), according to the manufacturer’s instructions. To extract RNA from cells, we used FACS to sort cells, or collected cells from culture plates, and placed them directly into vials containing lysis buffer. We isolated RNA using the Single Cell RNA Purification Kit (Norgen), according to the manufacturer’s instructions. We assessed the quality of total RNA using the 2100 Bioanalyzer system (Agilent Technologies) and the quantity using the Qbit RNA HS kit (Thermo Fisher). Total RNA was transcribed into complementary DNAs using SuperScript IV Reverse Transcriptase (Invitrogen). We performed qRT–PCR using RT2 qPCR primer sets (SABiosciences and Bio-Rad Laboratories, a list is provided in Supplementary Table 2) and SYBR-Green master mix (Bio-Rad Laboratories), on a PTC-200 thermocycler equipped with a Chromo4 detector (Bio-Rad Laboratories), and analyzed data using Opticon Monitor software (Bio-Rad Laboratories). Ct values were normalized to the mean of the Ct values obtained for the housekeeping genes Hsp90ab1, Hprt and Gapdh.
RNA sequencing
Librairies were prepared from total mRNA using the SMARTer Stranded Total RNA-Seq Kit v2-Pico Input Mammalian (Takara Bio), according to the manufacturer’s instructions. Library quality and quantity were assessed using the 2100 Bioanalyzer system (Agilent Technologies) and the Qbit dsDNA HS kit (Thermo Fisher). Sequencing was performed using Illumina NextSeq 500/550 High Output kit v2.5. The RNA-seq analysis was performed with Sequana 0.11.0 (https://github.com/sequana/sequana_rnaseq) built on top of Snakemake 6.1.1 (ref. 80). Briefly, reads were trimmed from adapters using cutadapt 3.4, then mapped to the genome assembly GRCm38 from Ensembl using STAR 2.7.3a. FeatureCounts 2.0.1 was used to produce the count matrix, assigning reads to features using corresponding annotation GRCm38_92 from Ensembl with strand-specificity information. Quality-control statistics were summarized using MultiQC 1.10.1 (ref. 81). Clustering of transcriptomic profiles was assessed using principal components analysis. Differential expression testing was conducted using DESeq2 library 1.22.2 (ref. 82). The normalization and dispersion estimation were performed with DESeq2 using the default parameters; statistical tests for differential expression were performed by applying the independent filtering algorithm. A generalized linear model was set in order to test for the differential expression between conditions. Raw P values were adjusted for multiple testing according to the Benjamini–Hochberg procedure, and genes with an adjusted P lower than 0.05 were considered differentially expressed. Gene set enrichment analysis was performed using Fisher’s exact test for the over-representation of upregulated genes.
Human tumors
We selected non-metastatic samples from three TCGA projects (RNA-seq analyses): skin cutaneous melanoma (SKCM), colon adenocarcinoma (COAD) and prostate adenocarcinoma (PRAD), all three in RKPM (Reads Per Kilobase Million) log2 + 1 transformed. We also selected one International Cancer Genome Consortium (ICGC) project, pancreatic ductal adenocarcinoma (PACA_AU, array-based analysis), retrieved from Bailey et al.83. We filtered out genes that had missing values in any of the samples or that were located in the Y chromosome locus. Each dataset was analyzed independently. For each dataset, ADAM12hi and ADAM12lo groups were defined according to their ADAM12 expression, either above or below median ADAM12 expression, respectively. Gene set enrichment analyses (GSEAs) were carried out using the fgsea R package. A Wilcoxon test was used to analyze mean differences in gene expression between the ADAM12hi and ADAM12lo groups, and the Wilcoxon test value was used to make the gene rankings for GSEA. GSEA parameters were were: fgseaMultilevel(pathways = (‘h.all.v7.0.symbols.gmt’, ‘c5.go.mf.v7.2.symbols.gmt’, ‘c2.cp.kegg.v7.1.symbols.gmt’, ‘c5.bp.v7.1.symbols.gmt’), stats = ‘Gene rank’, minSize = 5, maxSize = 500). GSEA results were displayed using the plotEnrichment function with default parameters from the fgsea package for GSEA curves. Genes accounting for a pathway being defined as enriched are refered to as ‘leading edges’. These genes were shared by all four datasets. Expression levels of the leading-edge genes in our samples were displayed as a heat map using the ComplexHeatmap R package.
Single-cell RNA-seq
Analysis of ADAM12 expression in single-cell RNA-seq was performed on previously published data84,85,86, and results were visualized using the Broad Institute’s Single Cell Portal (https://singlecell.broadinstitute.org/single_cell). For pancreatic ductal adenocarcinoma tumors, raw data from Peng et al.86 were analyzed using the Seurat (v.3.2) R package, with all functions ran with default parameters. Low-quality cells (<200 genes per cell, <3 cells per gene and >5% mitochondrial genes) were excluded from further steps. Cell-type identification was done using the SCINA R package, and gene markers from the MCP counter were used for stromal cells87. KRT19, CDH1, MUC1, SOX9 and EPCAM were used as marker genes for epithelial cells. Nonlinear dimensional reduction (t-SNE) was applied as described in ref. 86. The ‘Activated stroma’ signature score, as described by Puleo et al.65, was calculated for each cell using the default function AddModuleScore from Seurat.
Treatments and cell culture
MO5 melanoma cells were grown in RPMI medium supplemented with 10% FBS, 1% penicillin–streptomycin, G418 (2 mg mL–1) and hygromycin B (0.06 mg mL–1). To obtain CM, cells were isolated by FACS and seeded into 96-well flat-bottom plates in DMEM 10% FBS at 37 °C. We collected CM after 24–48 h for further experiments. For in vitro polarization of BMDMs, bone marrow cells were isolated and differentiated as previously described88, then incubated for 24 h in the indicated conditions. When indicated, BMDMs with specific deletion of AXL in myeloid cells were generated by crossing Axlfl/fl mice89 with LysMcre mice90. Axlfl/fl littermates were used as controls. For stimulation in vitro, OSM (5 ng mL–1), IL-1β (10 ng mL–1) or TGF-β (2 ng mL–1) were added on cells for the indicated times. Cell proliferation was measured with Cell Counting Kit-8 (CCK-8; Dojindo, no. 899650), according to the manufacturer’s instructions. To induce hypoxia, stromal cells were cultured in a hypoxic chamber (Whitley H35 Hypoxystation) in 1% oxygen for 72 h. The engulfing ability of macrophages was assessed by isolating the cells from tumors and incubating them for 30 min with Fluoresbrite Red microspheres (no. 18660–5, Polyscience) at 37 °C (or 4 °C for control condition), staining them with antibodies against F4/80 and analyzing them with Fortessa (BD Biosciences) and FlowJo software (Tristar). Gas6 was measured by ELISA, according to the manufacturer’s instructions (Mouse Gas6 DuoSet ELISA, R&D, no. DY986). The tumor extracellular pH was assessed using pH Microelectrodes (Fisher Scientific), following the manufacturer’s instructions.
Image quantification
The immunofluorescence signal was quantified using ImageJ and threshold processing on high-resolution tiles that were stiched to create mosaic images of the entire tumor section or on tumor fields, obtained with an AxioImager M1 fluorescence microscope (Zeiss) and ZEN software. Tumor hypoxia was detected using the Hypoxyprobe Plus Kit, following the manufacturer’s instructions, and the proportion of pimonidazole signal was measured by pixel quantification on mosaic images. The percentage of tumor perfused area was determined by normalizing Hoechst-positive area to the DAPI-positive area on mosaic images. Pericyte coverage was determined by measuring NG2/CD31 staining ratio by quantifying pixels on mosaic images of the total tumor section (all vessels in mosaic images were measured). Blood vessel width was measured using the Adobe Photoshop measurement tool. The proportion of AXL-P+ and cleaved-Casp3+ cells was measured by pixel quantification, and was normalized to F480- or DAPI-positive area, respectively. The tumor margin was identified as the peritumoral zone with a high density of PDPN+ or αSMA+ stromal cells.
Statistical analysis
We determined statistical significance using two-tailed unpaired Student’s t-test (with Welch’s correction for unequal s.d.) between two groups, Mann–Whitney U test if data were not normally distributed, and one- or two-way ANOVA or the Kruskal–Wallis test across multiple groups, as indicated in the legends. For one- or two-way ANOVA, we performed the Tukey or Sidak multiple-comparison correction, as determined on GraphPad Prism 9. When indicated, we compared conditions using a global Kruskal–Wallis test and then performed pairwise comparisons using a Wilcoxon test. P values from Wilcoxon tests were adjusted for multiple testing using the Bonferroni method. Unless otherwise specified, n represents the number of mice. No statistical method was used to predetermine sample size, which was chosen on the basis of prior experience and prior published studies with similar layout. No technical replicates across independent experiments were pooled in the datasets. Investigators were blind to the conditions of the experiments during data collection, and no animals or data points were excluded. Age- and sex-matched non-transgenic littermate mice were used as controls, and mice were randomly assigned in each group. Values are expressed as mean ± s.d. P < 0.05 was considered statistically significant.
Material availability
This study did not generate new unique reagents. Further information and requests for resources and reagents should be directed to the corresponding author, L.P.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The TCGA_PAAD, TCGA_SKCM, TCGA_COAD and TCGA_PRAD dataset can be accessed at https://portal.gdc.cancer.gov/. The ICGC_PAN_AU dataset can be accessed at: https://dcc.icgc.org/. scRNA-seq data of pancreatic cancer from Peng et al.86 are available from the Genome Sequence Archive (project PRJCA001063, https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA001063). scRNA-seq data from CRC84 and SKCM85 are accessible via the Gene Expression Omnibus (GEO) under accession codes GSE178341 and GSE115978, respectively. The genome GRCm38 (GCA_000001635.9) release 102 is accessible from Ensembl (http://www.ensembl.org/Mus_musculus/Info/Index). RNA-seq data generated in this study are deposited in NCBI’s Gene Expression Omnibus under accession codes GSE206794 (Depletion of ADAM12+ cells in MO5 tumors) and GSE206795 (ADAM12+ cells in MO5 tumors). Source data are provided with this paper.
Code availability
This paper does not report original code.
References
Schito, L. & Semenza, G. L. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2, 758–770 (2016).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Taylor, C. T., Doherty, G., Fallon, P. G. & Cummins, E. P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Invest. 126, 3716–3724 (2016).
Huber, V. et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 43, 74–89 (2017).
Prunier, C., Baker, D., Ten Dijke, P. & Ritsma, L. TGF-β family signaling pathways in cellular dormancy. Trends Cancer 5, 66–78 (2019).
Batlle, E. & Massague, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019).
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 (2015).
Laklai, H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505 (2016).
Davidson, S. et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020).
Dominguez, C. X. et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10, 232–253 (2020).
Costa, A. et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479 (2018).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Kraman, M. et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 330, 827–830 (2010).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Lee, J. J. et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl Acad. Sci. USA 111, E3091–E3100 (2014).
Cheng, H. W. et al. CCL19-producing fibroblastic stromal cells restrain lung carcinoma growth by promoting local antitumor T-cell responses. J. Allergy Clin. Immunol. 142, 1257–1271 (2018).
Jiang, H. et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J. Clin. Invest. 130, 4704–4709 (2020).
Chen, Y. et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39, 548–565 (2021).
Bhattacharjee, S. et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Invest. 131, e146987 (2021).
Peduto, L. et al. ADAM12 is highly expressed in carcinoma-associated stroma and is required for mouse prostate tumor progression. Oncogene 25, 5462–5466 (2006).
Frohlich, C. et al. Molecular profiling of ADAM12 in human bladder cancer. Clin. Cancer Res. 12, 7359–7368 (2006).
Le Pabic, H. et al. ADAM12 in human liver cancers: TGF-β-regulated expression in stellate cells is associated with matrix remodeling. Hepatology 37, 1056–1066 (2003).
Cireap, N. & Narita, D. Molecular profiling of ADAM12 and ADAM17 genes in human malignant melanoma. Pathol. Oncol. Res. 19, 755–762 (2013).
Veenstra, V. L. et al. ADAM12 is a circulating marker for stromal activation in pancreatic cancer and predicts response to chemotherapy. Oncogenesis 7, 87 (2018).
Dulauroy, S., Di Carlo, S. E., Langa, F., Eberl, G. & Peduto, L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 18, 1262–1270 (2012).
Roy, R. & Moses, M. A. ADAM12 induces estrogen-independence in breast cancer cells. Breast Cancer Res. Treat. 131, 731–741 (2012).
Ma, B. et al. ADAM12 expression predicts clinical outcome in estrogen receptor-positive breast cancer. Int. J. Clin. Exp. Pathol. 8, 13279–13283 (2015).
Cheon, D. J. et al. ADAM12 is a prognostic factor associated with an aggressive molecular subtype of high-grade serous ovarian carcinoma. Carcinogenesis 36, 739–747 (2015).
Du, S. et al. ADAM12 is an independent predictor of poor prognosis in liver cancer. Sci. Rep. 12, 6634 (2022).
Ten Hoorn, S. et al. Serum-based measurements of stromal activation through ADAM12 associate with poor prognosis in colorectal cancer. BMC Cancer 22, 394 (2022).
Mino, N. et al. A disintegrin and metalloprotease 12 (ADAM12) is a prognostic factor in resected pathological stage I lung adenocarcinoma. J. Surg. Oncol. 100, 267–272 (2009).
Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Djurec, M. et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc. Natl Acad. Sci. USA 115, E1147–E1156 (2018).
Gomez-Chou, S. B. et al. Lipocalin-2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer Res. 77, 2647–2660 (2017).
Nakamura, I. et al. Lipocalin2 as a plasma marker for tumors with hypoxic regions. Sci. Rep. 4, 7235 (2014).
Gonzalez-Avila, G., Sommer, B., Garcia-Hernandez, A. A. & Ramos, C. Matrix metalloproteinases’ role in tumor microenvironment. Adv. Exp. Med. Biol. 1245, 97–131 (2020).
Pastorekova, S. & Gillies, R. J. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 38, 65–77 (2019).
Calcinotto, A. et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 72, 2746–2756 (2012).
Pilon-Thomas, S. et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 76, 1381–1390 (2016).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Uutela, M. et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood 104, 3198–3204 (2004).
Ye, L. & Jiang, W. G. Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett. 380, 586–597 (2016).
Schwoerer, S. et al. Hypoxia potentiates the inflammatory fibroblast phenotype promoted by pancreatic cancer cell-derived cytokines. Cancer Res. 83, 1596–1610 (2023).
Mello, A. M. et al. Hypoxia promotes an inflammatory phenotype of fibroblasts in pancreatic cancer. Oncogenesis 11, 56 (2022).
Myers, K. V., Amend, S. R. & Pienta, K. J. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol. Cancer 18, 94 (2019).
Vennin, C. et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 10, 3637 (2019).
Saatci, O. et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 11, 2416 (2020).
Shen, Y. W., Zhou, Y. D., Chen, H. Z., Luan, X. & Zhang, W. D. Targeting CTGF in cancer: an emerging therapeutic opportunity. Trends Cancer 7, 511–524 (2021).
Krishnamurty, A. T. et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 611, 148–154 (2022).
Siegel, P. M. & Massague, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 3, 807–821 (2003).
Gaengel, K., Genove, G., Armulik, A. & Betsholtz, C. Endothelial–mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 (2009).
Morioka, S., Maueroder, C. & Ravichandran, K. S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50, 1149–1162 (2019).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Werfel, T. A. & Cook, R. S. Efferocytosis in the tumor microenvironment. Semin Immunopathol 40, 545–554 (2018).
Fan, Z. et al. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood 107, 1342–1351 (2006).
Johansson-Percival, A. et al. Intratumoral LIGHT restores pericyte contractile properties and vessel integrity. Cell Rep. 13, 2687–2698 (2015).
Zagorska, A., Traves, P. G., Lew, E. D., Dransfield, I. & Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 15, 920–928 (2014).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eaak9670 (2017).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Le Pabic, H. et al. Involvement of the serine/threonine p70S6 kinase in TGF-β1-induced ADAM12 expression in cultured human hepatic stellate cells. J. Hepatol. 43, 1038–1044 (2005).
Misemer, B. S. et al. Expression of FAP, ADAM12, WISP1, and SOX11 is heterogeneous in aggressive fibromatosis and spatially relates to the histologic features of tumor activity. Cancer Med. 3, 81–90 (2014).
Ebbing, E. A. et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc. Natl Acad. Sci. USA 116, 2237–2242 (2019).
Puleo, F. et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 155, 1999–2013 e1993 (2018).
Jones, S. A. & Jenkins, B. J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 18, 773–789 (2018).
Cully, M., You, H., Levine, A. J. & Mak, T. W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 6, 184–192 (2006).
Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Lewis, C. E., Harney, A. S. & Pollard, J. W. The multifaceted role of perivascular macrophages in tumors. Cancer Cell 30, 18–25 (2016).
Clever, D. et al. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 166, 1117–1131 (2016).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Triner, D. & Shah, Y. M. Hypoxia-inducible factors: a central link between inflammation and cancer. J. Clin. Invest. 126, 3689–3698 (2016).
Schonig, K., Schwenk, F., Rajewsky, K. & Bujard, H. Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 30, e134 (2002).
Leveen, P. et al. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 100, 560–568 (2002).
Celluzzi, C. M., Mayordomo, J. I., Storkus, W. J., Lotze, M. T. & Falo, L. D. Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 183, 283–287 (1996).
Greenberg, N. M. et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA 92, 3439–3443 (1995).
Hanahan, D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315, 115–122 (1985).
DuBois, J. C., Ray, A. K., Davies, P. & Shafit-Zagardo, B. Anti-Axl antibody treatment reduces the severity of experimental autoimmune encephalomyelitis. J. Neuroinflammation 17, 324 (2020).
Koster, J. & Rahmann, S. Snakemake—a scalable bioinformatics workflow engine. Bioinformatics 28, 2520–2522 (2012).
Ewels, P., Magnusson, M., Lundin, S. & Kaller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752 (2021).
Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997 (2018).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Becht, E. et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 17, 218 (2016).
Pineda-Torra, I., Gage, M., de Juan, A. & Pello, O. M. Isolation, culture, and polarization of murine bone marrow-derived and peritoneal macrophages. Methods Mol. Biol. 1339, 101–109 (2015).
Schmid, E. T. et al. AXL receptor tyrosine kinase is required for T cell priming and antiviral immunity. eLife 5, e12414 (2016).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Acknowledgements
We thank the Cytometry Platform at the Institut Pasteur; the Biomics Olatform (C2RT, Institut Pasteur; supported by France Génomique (ANR-10-INBS-09) and IBISA) and the animal facility. We thank B. Charbit, G. Faucher and A. Lepelletier for excellent technical assistance, and C. Leclerc for the MO5 cell line. S.D.C. was funded by La Ligue contre le Cancer and Institut Pasteur. A.O. was funded by a doctoral fellowship from Le Ministère de l’Enseignement Supérieur et de la Recherche. The lab has received funding from the European Research Council (ERC) Consolidator grant 648428-PERIF (to L.P.), INSERM (to L.P.), l’Institut National du Cancer (INCa #PLBIO21-264) (to L.P.), and Institut Pasteur (to L.P.).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and validation, S.D.C. and L.P.; experiments and data analysis, S.D.C., D.C.G., A.O., M.S., L.P.; RNA-seq analysis, H.V. and R.L.; Bioinformatic analysis of human tumors, J.R. and C.B.; providing material, J.T.; writing—original draft, S.D.C. and L.P.; funding acquisition, L.P.; supervision, L.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks David Ting, Carla Rothlin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Depletion of ADAM12+ MSCs restores tumor immunity.
(a) Immunofluorescence staining of the indicated markers in MO5 tumors. Scale bar, 50 µm. (b) Immunofluorescence staining of PDPN and GFP in MO5 tumors growing in GFP+ mice, as in Fig. 1b. Scale bar, 100 µm. (c) Immunofluorescence staining of PDPN+ cells in normal skin. Scale bar, 100 µm. (d) Absolute numbers of GFP+ cells at the indicated days after tumor inoculation, as in Fig. 1c. (e) Expression of Adam12, measured by qRT-PCR, in the indicated populations isolated by FACS from MO5 tumors; n = 6 from 2 independent experiments. (f) FACS plot and percentages of GFP+ cells in the indicated populations from Fig. 1c. (g-i) Immunofluorescence staining of the indicated markers in MO5 tumors growing in GFP+ mice (g,h) or GFP– littermates (i). White arrows indicate GFP+ cells; red arrows indicate pericytes; white arrowheads indicate the vBM. Scale bar, 10 µm. (j) Expression of human DTR (HBEGF) measured by qRT-PCR in the indicated conditions; n = 7, except for DTR(n = 9). (k) Expression of Adam12 detected by RNAscope in tumor sections from mice treated as in Fig. 1d; n = 6(DTR)-8(Ctrl) fields from 2 independent experiments. Scale bar, 50 µm. (l,m) Individual animal growth curves from Fig. 1d (l) and Fig. 1e (m). (n) Percentage of tumor infiltrating CD4+ T cells (n = 9 Ctrl, n = 8 DTR), neutrophils (n = 9 Ctrl, n = 8 DTR), eosinophils (n = 13 Ctrl, n = 11 DTR), DCs (n = 13 Ctrl, n = 11 DTR), M-MDSCs (n = 8 Ctrl, n = 6 DTR), G-MDSCs (n = 8 Ctrl, n = 6 DTR), macrophages (n = 13 Ctrl, n = 11 DTR), Tregs (n = 5 Ctrl, n = 7 DTR), measured by FACS. (o,p) Percentage of CD8+ T cells (n = 6 Ctrl, n = 4 DTR), IFNγ+ CD8+cells (n = 9 Ctrl, n = 7 DTR) (o) and the indicated fibroblastic populations (n = 4 Ctrl, n = 3 DTR) (p) in the tumor draining lymph node (LN), as measured by FACS. (q) Tumor growth curves from the indicated mice (n = 11, DTR + I gG; n = 9, DTR + anti-CD8), from 2 independent experiments. Left: average tumor volume and Right: individual animal growth curve. In l,m,q, the x axis represents days after tumor inoculation. In a-c,g-i one representative image of at least 3 independent experiments is shown. FRC: fibroblastic reticular cells; FDC: follicular dendritic cells; MRC: marginal reticular cells; TRC: T-zone FRCs. Statistics were calculated using two-tailed, unpaired Student’s t-test (e,n,o), Kruskal-Wallis test (j), two-tailed Mann-Withey test (k) or two-way ANOVA (q). All quantitative data are presented as mean values +/– SD.
Extended Data Fig. 2 Immune and vascular remodeling of the TME.
(a) Percentage of CD3+ T cells in proximity to PDPN+ stromal cells in mice treated as in Fig. 2a. Measurements were assessed in 3 fields/tumor from independent experiments (n = 3). (b) Absolute numbers of PDPN+ PDGFRα+ cells (gated CD45–CD31–), measured by FACS, in MO5 tumors growing in mice treated as in Fig. 2a; n = 12(Ctrl)–14(DTR) from 3 independent experiments. (c) Percentage of the indicated immune populations, measured by FACS, infiltrating MO5 tumors in WT mice treated with U-104 inhibitor; from independent experiments (n = 5). (d) Hypoxic zones (identified by pimonidazole staining) in frozen sections of MO5 tumors treated as in c (n = 4); scale bar, 100 µm. (e) Immunofluorescence staining of NG2 and CD31 in frozen sections of MO5 tumors from mice treated as in Fig. 2i. Scale bar, 200 µm. T: tumor. In d,e, one representative image of independent experiments is shown. Statistics were calculated using two-tailed, unpaired Student’s t-test. Data are presented as mean values +/– SD. ns: not significant.
Extended Data Fig. 3 Stromal remodeling of the TME.
(a) FACS gating strategy for S1, S2 and S3 stromal populations from MO5 tumors. (b) Normalized expression of the indicated genes in stromal populations from melanomas single cells RNAseq9. (c) Expression of the indicated genes, measured by qRT-PCR, in S1, S2 and S3 isolated from MO5 tumors as shown in a (n = 3). (d) Percentage of the indicated stromal populations, measured by FACS, in MO5 tumors from ADAM12-DTR+ (DTR) and DTR– littermates (Ctrl) treated with DT as in Fig. 2a (n = 4). (e) Normalized expression of Adam12 from single cells RNAseq of melanomas and normal skin9. (f) FACS plot and percentages of GFP+ cells in the total stromal fraction from MO5 tumors. One representative experiment from independent experiments is shown (n = 7). (g) Expression of the indicated genes, measured by qRT-PCR, in stromal cells treated as indicated; n = 3. Gating strategy for stromal populations S1: PDGFRαHigh PDPNHigh CD34High DPP4High Ly6cHigh; S2: PDGFRα+ PDPN+ CD34Mid/Low DPP4Low/–; S3: PDGFRαLow PDPNLow CD34Low DPP4–. Statistics were calculated using two-tailed, unpaired Student’s t-test (d,g). Data are presented as mean values +/– SD.
Extended Data Fig. 4 Immune-stroma crosstalk and Tgfbr2 signaling in ADAM12+ cells.
(a) Violin plot of Lrrc15 from RNASeq data in Fig. 3a (n = 4). (b) Immunofluorescence staining of Ki67 and GFP in melanomas growing in ADAM12-GFP mice. Scale bar, 50 µm. (c) Expression of the indicated genes, measured by qRT-PCR, in the indicated populations isolated by FACS from MO5 tumors at day 12, n = 3 from independent experiments. Macrophages (M), tumor cells (MO5), endothelial cells (EC). (d) Expression of Adam12, measured by qRT-PCR, in ADAM12– PDGFRα+ cells isolated from MO5 tumors, treated as indicated (n = 3). (e) Expression of Gas6, measured by qRT-PCR, in stromal cells cultured in indicated conditions (n = 3). (f) Normalized expression of Tgfb1 in normal skin and melanomas (from single cells RNAseq dataset12). (g) Expression of the indicated genes, measured by qRT-PCR, as in c. n = 3 except n = 6 (stroma). (h) Violin plots of Tgfbr1 and Tgfbr2 from RNASeq data in Fig. 3a (n = 4). (i) Expression of Tgfbr2, measured by qRT-PCR, in GFP+ and GFP– stromal cells isolated by FACS from MO5 tumors (n = 4). (j) Strategy for inducible depletion of Tgfbr2 in ADAM12+ cells. (k) Experimental set up for l-p. (l) Tumor growth curves from ADAM12-tTA2-CreTgfbr2 mice (Tgfbr2fl, n = 8) and littermate mice (Ctrl, n = 6) treated as indicated in k. Left, average tumor volume, and right, individual animal growth curves, from 2 independent experiments. The x axis represents days after tumor inoculation. (m) Immunofluorescence staining of PDPN and CD3 in tumor sections in the indicated conditions. Right, quantification of tumor infiltrating T cells was performed on n = 6(Ctrl)–7(Tgfbr2fl) fields. Scale bars, 100 μm. (n) Expression of Vegfa, measured by qRT-PCR, in macrophages isolated from tumors. n = 7(Ctrl)–6(Tgfbr2fl) from 2 independent experiments. (o) Immunofluorescence staining of NG2 and CD31 in tumor sections from mice treated as in k; n = 7(Tgfbr2fl)–9(Ctrl) fields. Scale bars, 100 μm. (p) Expression of the indicated transcripts, measured by qRT-PCR, in tumor stromal cells isolated by FACS. n = 3 from independent experiments. In b,e, one representative experiment out of 3 is shown. Statistics were calculated using two-tailed, unpaired Student’s t-test (d,e,i,m-p) or two-way ANOVA (l). Data are presented as mean values +/– SD.
Extended Data Fig. 5 ADAM12+ cells promote efferocytosis.
(a) Immunofluorescence staining of CD3+ T cells and ADAM12+ cells in MO5 tumors margin. Arrowheads indicate T cells infiltrated zones, and arrows indicate ADAM12+ cells. Scale bar, 100 µm. (b) Percentage of tumor stromal cells expressing ADAM12, DPP4 or αSMA in close proximity to AXL-P+ macrophages, as in Fig. 4b (n = 4, except for DPP4+ cells, n = 7), from 2 independent experiments. (c) Expression of Gas6, measured by qRT-PCR, in macrophages (M), T cells, tumor cells (MO5) and stromal cells isolated from MO5 tumors, n = 3. (d) Efferocytosis assay in BMDM, and macrophages or PDPN+PDGFRα+ stromal cells isolated from MO5 tumors (n = 3). AC= apoptotic cells (MO5). (e) Expression of the indicated genes, measured by qRT-PCR in BMDM following efferocytosis (n = 4). (f) Immunofluorescence staining of the indicated markers in MO5 tumors depleted from ADAM12+ cells. Scale bar, 50 µm. Arrowheads indicate cleaved caspase 3+ apoptotic cells. BV= blood vessels; SC= stromal cells. (g) Phagocytic capacity of macrophages isolated by FACS from MO5 tumors growing in ADAM12-DTR or Ctrl littermate mice + DT, as in Fig. 4g; n = 6(DTR)-7(Ctrl) from two independent experiments. (h) Expression of Gas6, measured by qRT-PCR in MO5 tumors growing in mice treated as in Fig. 4g; n = 10(DTR)-12(Ctrl) from 3 independent experiments. In a,f, one representative image from 3 independent experiments is shown. Statistics were calculated using one-way ANOVA (b) or two-tailed, unpaired Student’s t-test (e,g,h). All quantitative data are presented as mean values +/– SD.
Extended Data Fig. 6 PDPN+ stroma and macrophages localization.
Immunofluorescence analysis of PDPN+ stromal cells (red) and F480+ macrophages (green) in frozen sections of tumors isolated from TRAMP mice (a) and RipTag2 mice (b). One representative image from 3 independent experiments is shown. Scale bar, 50 µm. DAPI stains nuclei.
Extended Data Fig. 7 The progeny of ADAM12+ cells in spontaneous tumor models.
(a) Immunofluorescence staining of GFP and the indicated markers in RipTag (n = 4) and TRAMP (n = 3) tumors. Right, absolute numbers of GFP+ cells measured by FACS. (b) Immunofluorescence staining of YFP and the indicated markers in MO5 tumors from ADAM12-tTA-CreYFP mice. Right, frequency and absolute numbers of YFP+ cells measured by FACS in normal skin (n = 2) and tumor (n = 4). (c) Expression of the indicated transcripts, measured by qRT-PCR, in GFP+ and YFP+ cells isolated from MO5 tumors growing in ADAM12-GFP mice or ADAM12-tTA-CreYFP mice, respectively; n = 4, except for Angpt2 and Acta2 YFP+ (n = 6) from independent experiments. (d) Immunofluorescence staining of YFP and the indicated markers in prostate and pancreatic tumors from the indicated mice. (e) Absolute numbers of YFP+ cells measured by FACS on independent mice, as indicated in Fig. 5g. (f) Immunofluorescence staining of fetal YFP+ cells in adult non-tumoral skin, prostate and pancreas. (g) Volume of pancreatic tumors in RIPTag/DTR or RIPTag2 littermate mice (Ctrl) treated with DT from weeks 11 to 14; n = 7(Ctrl)–11(DTR) from independent experiments. (h) Immunofluorescence staining of PDPN and CD3 in tumor sections from mice treated as in g. One representative image is shown of independent experiments (n = 4). (i) Absolute numbers of tumor infiltrating CD3+ T cells. Quantifications were performed on n = 10 images from 6 mice per group. (j) Percentage of PDPN+ PDGFRα+ cells, measured by FACS, in RIP-Tag2 tumors from mice treated as in g; n = 12(Ctrl)–15(DTR) from 6 independent experiments. (k) Distribution of PDPN+ cells within RIPTag tumors, in mice treated as in g; n = 9(Ctrl)-11(DTR) from 3 independent experiments. (l) Immunofluorescence staining of the indicated markers in tumor sections of mice treated as in g; quantification left, n = 13(Ctrl)–20(DTR) and right, n = 9(Ctrl)–8(DTR) fields. (m) Immunofluorescence staining of ICAM1, CD45 and CD31 in tumor sections of mice treated as in g; n = 11(Ctrl)-12(DTR) fields. (n) Tumor perfusion, as detected by Hoechst 33342 staining, in tumor sections from mice treated as in g; n = 9(DTR)-11(Ctrl) fields. In a,b,d,f, representative images of at least 3 independent experiments are shown. Statistics were calculated using two-tailed, unpaired Student’s t-test, except for c (Mann–Whitney test). All quantitative data are presented as mean values +/– SD. Scale bars, 50 μm, 100 µm (f,h), and 200 μm (n).
Extended Data Fig. 8 Characterization of ADAM12+ cells in human tumors.
(a) Expression of ADAM12 transcript in single-cell RNAseq from human colorectal cancer patients84 (N = 62 patients, total 371,223 cells). Clustering = All cells (tSNE); On x axis, all cell subsets from the tumors are represented (88), and the major clusters expressing ADAM12 are highlighted (annotation from Pelka et al.84; Pericyte_cS19, max: 3.314; q3: 1.08525, median: 0; CAF_cS28, max: 4.485, q3:1.50125, median: 0.558). (b) Expression of ADAM12 transcripts in single-cell RNAseq from melanoma patients85 (N = 31 melanoma tumors, total 39744 cells). Clustering = Non-malignant cells from melanoma tumors (CAF, max: 5.2823, q3: 3.0613, mean: 1.49153, median: 0). (c) Expression of ADAM12 transcripts in single-cell RNAseq from human PDAC86 (N = 24 primary untreated PDAC tumors, 41986 cells). Clustering= All cells (see Methods). (d) tSNE representation of ADAM12 expression in human PDAC as described in Peng et al.86 (top panel) and transcriptional signature of activated stromal PDAC subtype as described in Puleo et al.65 (lower panel). Two-sided Pearson’s Correlation Coefficient (R) was measured between ADAM12 expression and the activated stromal subtype signature (displayed in the tSNE panel). (e) Expression of the indicated transcripts in scRNAseq dataset from d. (f) Clinicopathologic characteristics of patients from the TCGA_PRAD project studied in Fig. 6. Patients (N = 455) were grouped according to their expression of ADAM12, as described in Methods. The associations were tested using two-sided Pearson chi2 test for categorical variables and the Mann–Whitney U test for continuous variables. Pathological classification: T – Extent of the primary tumor; N – Absence or presence and extent of regional lymph node metastases; M – Absence or presence of distant metastases. In a,b, data were visualized on the Single Cell portal of The Broad Institute of MIT and Harvard (https://singlecell.broadinstitute.org/single_cell).
Extended Data Fig. 9 Gating strategy for stromal cells.
Flow cytometry gating strategy for PDPN+PDGFRα+ stromal cells in early stage MO5 tumor.
Supplementary information
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Carlo, S.E., Raffenne, J., Varet, H. et al. Depletion of slow-cycling PDGFRα+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis. Nat Immunol 24, 1867–1878 (2023). https://doi.org/10.1038/s41590-023-01642-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01642-7