Abstract
Purpose
Analysis of microstructural alterations of corneal and limbal epithelial cells in healthy human corneas and in other ocular conditions.
Patients and methods
Unilateral eyes of three groups of subjects include healthy volunteers (G1, n=5), contact lens wearers (G2, n=5), and patients with dry eyes (G3, n=5) were studied. Imaging of basal (BC) and intermediate (IC) epithelial cells from central cornea (CC), corneal limbus (CL) and scleral limbus (SL) was obtained by in vivo confocal microscopy (IVCM). An appropriate image analysis algorithm was used to quantify morphometric parameters including mean cell area, compactness, solidity, major and minor diameter, and maximum boundary distance.
Results
The morphometric parameters of BC and IC demonstrated no significant differences (P>0.05) between groups. Comparison between three corneal locations (CC, CL, and SL) within the groups showed significant differences (P<0.05) with mean values of cell area, compactness, solidity, and major and minor diameter of BC that increase from CC to limbus. The BC were round and regular in the central cornea (P<0.05) compared with CL and SL.
Conclusions
IVCM enables high-quality confocal images from central corneal and limbal epithelium. This quantitative study demonstrated morphological differences in the basal and intermediate epithelium between limbus and central cornea, and found no differences between contact lens wearers, dry eyes, and normal subjects.
Similar content being viewed by others
Introduction
The X, Y, Z hypothesis1 explains cell mechanism that is essential for the renewal and maintenance of the corneal epithelium. This hypothesis proposes that the loss of corneal epithelial surface cells (Z) can be maintained by the proliferation of basal epithelial cells (X), and the centripetal movements of the peripheral epithelial cells (Y). By utilizing this mechanism, it is also possible to categorize both disease and therapies according to the specific component involved.1 Therefore it is vital to understand the cellular structures of both central and limbal epithelial cells in normal and in various corneal disease conditions.
Non-invasive in vivo confocal imaging2 of the living cornea is a novel clinical technique for the study of corneal cellular morphology. Earlier, the systematic approach in acquiring high quality and reproducible confocal images and subsequent precise cell quantification were the major challenges in the field of confocal microscopy. Today, due to the significant advancements in the optical properties of the confocal microscopy, the image quality has been dramatically improved and the digital computing systems enable compression and storage of huge data sets for the further processing of the images for the quantification of cellular structural alterations.3, 4, 5
Recently, in vivo confocal microscopy (IVCM) was used in studying the anatomical structures of the corneal and limbal epithelium4 particularly the palisades of Vogt (POV) including inter-palisadal epithelial rete pegs. POV6 are clinically visible radially oriented fibrovascular ridges observed along the upper and the lower corneoscleral limbus. Inter palisadal region serve as a repository for corneal epithelial cells. These interesting morphological structures of the limbus also thought to provide the microenvironment or niche for progenitor or limbal stem cells.7, 8 Any degree of disease or destruction to corneoscleral limbus leads to the formation of opaque conjunctival sheet of cells on the cornea causing disruption of smooth and clear optical surface leading to visual impairment or blindness.
In vivo corneal confocal studies have also quantified the characteristic features of the central and limbal epithelial cells using inbuilt or commercial software tools that require manual intervention and identification of cells, which may cause random and/or systemic error. To minimize these errors, we have used a fully automated software base5 with advanced image acquisition protocols and segmentation routines for the quantification of features of the epithelial cells.
The present work is the further step on the clinical application of the developed quantitative methods of the cellular alterations.5 Here we have studied two different corneal conditions that include contact lens wearing subjects9, 10 who are prone to develop mechanical or hypoxia-related corneal changes and subjects with dry eyes where corneal epithelium is stressed due to tear film insufficiency and frequent application of artificial tear drops.11 The purpose of this study was to quantify and compare the morphological features of the central and limbal epithelium between normal and other corneal conditions.
Materials and methods
In vivo confocal microscopy
IVCM was performed that works on the principle of Heidelberg Retina Tomograph II in combination with Rostock Cornea Module12 (HRT II/RCM; Heidelberg Engineering, Heidelberg, Germany). As already described by our group,13 this system enables both automated internal and manual external z-scan device to move the focal plane on to the corneal surface and thus allow imaging of cell layers at any depth with an axial resolution of about 1 μm. High magnification was achieved by using an immersion lens with a short focal length and a high numerical aperture (Achroplan × 63W/NA 0.95/AA 2.00 mm, 670 nm/Fa C Zeiss). The distance between corneal surface and the objective lens was kept stable using a single-use contact element (Tomocap; Heidelberg Engineering, Heidelberg, Germany) with a planar surface with a refractive index of 1.49.
Image identification
We have used the internal volume scan modality with a back and forth oscillation option that allows automatic image acquisition of up to 160 continuous optical sections with three cycles of oscillation (160 × 3=480 scans). Image acquisition from central cornea and inferior limbus (includes corneal limbus and scleral limbus) produced 960 (480 scans × 2) serial images of full-thickness corneal and inferior limbal epithelium from single eye including all the cell layers. The distance between two optical sections was about 0.4 μm, as determined earlier.5 From 960 images, a single cycle (160 images) of best sequence has been selected from each examined eye. For the automatic cell analysis, two consecutive optical sections of intermediate cell layer and two consecutive sections of basal cell layer were selected from each sequence. Therefore total of 60 images (4 images × 15 subjects=60) was processed for automated cell analysis. The scanned area of an image was 400 × 400 μm, 8-bit grey scale with 384 × 384 pixels.
Automatic cell segmentation
Automatic cell segmentation process involved several crucial steps.5 Initially, the images were magnified by a factor of 4 and the central 60% of overall dimension was used for cell segmentation to exclude image borders with low contrast. Inverted images were smoothed and enhanced using different image filters, finally image artefacts and incorrectly segmented cells were deleted for quantification. The segmented cells were labelled and number of their pixels was counted to measure different cell parameters. The quantified cell parameters (Table 1) include cell area, compactness, solidity, major and minor cell radius, and maximal boundary distance of both intermediate and basal cell layers. The compactness and solidity are the shape factors that explain the shape and regularity of the cells, whereas other parameters measure the size of the epithelial cells.
Subjects
Three different groups of subjects (15 with each group having 5) were studied including normal or control group (G1), contact lens wearers (G2), and the subjects with dry eyes (G3). Subjects from G1 and G2 were the volunteers from University of Rostock while group 3 was the patients followed up in the regular outpatient department of the Eye clinic, University of Rostock. Patient demographics and the clinical presentation have been summarized in Table 2. Oral informed consent was taken from all subjects before participating in the study and followed the tenets of the Declaration of Helsinki. Detailed contact lens history has been collected from all subjects from G2 before the examination. Table 3 describes the history of contact lens wear. The IVCM was performed on the unilateral eyes of all 15 subjects at three different corneal locations including the central cornea (CC), corneal (CL) and scleral (SL) sides of the inferior limbus.
Statistical analysis
All data were stored and analysed using the SPSS statistical package 15.0 (SPSS Inc. Chicago, IL, USA). The statistics computed included mean and SD’s of continuous variables, frequencies, and relative frequencies of categorical factors. Analysis of variance (ANOVA) was used to analyse the differences between independent group means and LSD-post hoc tests are realized for situations in which a significant omnibus F-test is obtained and additional exploration of the differences among means is needed to provide specific information, on which means are significantly different from each other. For comparison between different locations of cornea within group samples repeated measures ANOVA was applied. All P-values resulted from two-sided statistical tests and P≤0.05 was considered to be significant.
Our trial was designed as a pilot study with an objective to provide sufficient evidence that a larger definitive trial can be undertaken and, at times, to provide a preliminary assessment of benefit. Therefore, no statistical sample size estimations were made. The focus was on descriptive statistics and estimations. Of course, achievement of statistical significance is of value but because pilot studies are usually underpowered no significant results are to be interpreted in a strictly explorative way.
Results
The mean age of control group (G1), contact lens wearing group (G2), and dry eye subjects (G3) was 36.2±11, 32.0±4, and 50.2±28, respectively, with the total mean age of 39.4±9 years.
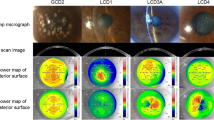
Figure 1a demonstrates the confocal microscopic images of intermediate (wing cells) and basal cells at three corneal locations (CC, central cornea; CL, corneal limbus; and SL, scleral limbus). An example from each group presented in this figure clearly demonstrates the anatomical structures of palisades of Vogt (POV) and inter palisadal rete ridges. Briefly, in the normal subject, oval-shaped POV are detected at the corneal limbus in both cell layers (intermediate and basal) while ridge-like extensions were present at the scleral limbus. Similarly, oval-shaped and ridge-like POV were also noted in contact lens wearer, however, POV were slightly different in appearance having dumb-belled shape. The dry eye subject had demonstrated ridge-like extensions with sharp edges. Inter palisadal rete ridges, also known as rete pegs, comprises several layers of compact cells of darker cytoplasm with bright cell border. Other microscopic observations14 include basal cells with hyperreflective nucleus and scattered dendritic cells in contact lens wearers and sclerocorneal region with bleeding blood vessels, absence of POV and hyperreflective network-like structures interfering with the clear view of limbal cells in moderate to severe dry eye subjects. These noticeable epithelial morphological changes on confocal microscopy of these study subjects have already been demonstrated and discussed in detail by our group.14
(a) In vivo confocal microscopy—an example from each group of subjects demonstrating basal and intermediate cell layers in the central cornea and limbus. CC, central cornea; CL, corneal limbus; SL, scleral limbus; BC, basal cells; IC, intermediate cells; arrows, ridge-shaped palisades of Vogt (POV); asterisk, oval-shaped palisades of Vogt (POV); arrow heads, inter-palisadal epithelium, the rete pegs. (b) Mean±SD graphs for cell area (i), compactness (ii), solidity (iii), major diameter (iv), minor diameter (v), and maximum boundary distance (vi) of basal cells at different corneal locations, comparing between groups. CC, central cornea; CL, corneal limbus; SL, scleral limbus. (c) Mean+SD graphs for cell area (i), compactness (ii), solidity (iii), major diameter (iv), minor diameter (v), and maximum boundary distance (vi) of intermediate cells at different corneal locations, comparing between groups. CC, central cornea; CL, corneal limbus; SL, scleral limbus.
Morphometry comparison study between groups (Figure 1b and c) found no significant differences in any of the measured parameters of both types of cells (BC and IC). Morphometry comparison study (Figure 1b and c) within the groups found significant differences between three measured corneal locations (CC, CL, and SL). Mean cell area of BCs in corneal limbus (P=0.005) and scleral limbus (P=0.001) in G1 and corneal limbus (P=0.006) in G3 were significantly greater in comparison with central cornea. Mean cell compactness of BCs and ICs demonstrated a gradual increase in compactness from CC to CL and to SL. It means cells at the central cornea are close to round in shape and it becomes slightly irregular towards periphery. This difference in compactness was significant (P<0.05) with BCs between CC and CL in G2, with ICs between CL and SL in G1, and between CC and CL in G3. Mean cell solidity measures the regularity of the cell boundary from its convex hull. Numerical value 1 indicates lowest solidity, the increasing value indicate increase in irregularity of cell boundary. Significant difference was found in mean cell solidity of BCs between CC–CL and CC–SL in G1, and between CC and CL in G3. Mean cell major diameter of basal cells at the peripheral cornea were found to be larger in size in comparison with central cornea. The P-value was <0.05 for BC between CC–CL and CC–SL in G1 and G2, and between CC and CL in G3. Mean cell minor diameter of basal cells was measuring slightly larger with P<0.05 between CC–CL and CC–SL only in G1. Mean cell maximum boundary distance of BC showed significant difference (P<0.05) between CC–CL and CC–SL in G1 and G2, and between CC–CL in G3.
Discussion
The image acquisition of the corneal epithelial cells and limbus has always been challenging in the field of confocal microscopy due to its critical anatomical position in the eye. The Rostock IVCM made it easier and successful in capturing high-quality serial images of the central and limbal epithelium suitable for both qualitative and quantitative analysis.
In the present study, 12 out of 15 subjects demonstrated POV with interpalisadal rete ridges. However, the configuration of these structures was highly variable between subjects demonstrating linear finger like, oval- or circular-shaped POV, similar structures have also been reported in the literature.4 In 80% of our study subjects, the POV could be easily detected at the scleral side of the limbus at the level of basal epithelium that extending into the stromal layers. Tightly packed epithelial basal cells at the depth of 100 μm in-between rete ridges were taken for quantitative analysis. The acquisition of POV was critical and it was invisible or unclear in three subjects with dry eyes. The possible reasons could be the older age, relatively less-pigmented eyes, and/or the severity of the disease condition. Supporting to this, Patel et al explained that the POV are difficult or not visualized in non-pigmented subjects and in older subjects.15 Studies4, 6 have found that POV to be predominantly visible along the superior and inferior limbus and it seem to be more regular and prominent at the lower limbus. Therefore, we have examined corneal and scleral sides of the inferior limbus in our study subjects. The scleral side of the limbal cells (SL) in dry eye subjects were less clear or difficult to view due to highly reflective network-like structures and thus excluded from quantitative analysis.
The results from central corneal basal and intermediate cells of normal study group seem to be consistent to our results previously reported5 where a similar quantitative method has been used on different set of study subjects. We have demonstrated the smallest basal cells in the CC that increase in size towards limbus (CL>SL) in all three groups (G1, G2, and G3), concur with the findings of Patel et al15 who reported lower epithelial cell density or increased cell size at the limbal palisades compared with CC in healthy individuals, however, density was greatest in the CL. Our findings regarding the cell diameter and cell area were in contrast with the results from Miri et al4 and others13, 16, 17 who have reported smaller cells with greater density at the limbal rete pegs. Romano et al16 have also demonstrated that the smallest cells of the limbal epithelium possess lowest cytoplasmic granularity, implying the extent of cell differentiation and suggested that this characteristic feature may help in isolating stem cells. Romano et al16 have also discussed possible variation in the extent of POV and cell density at different quadrants other than the superior limbus. The possible reasons for these contrasting results could be the location and depth of the limbus evaluated, smaller number (five in each group) of study population in each group in the present study, and the variation in quantitative methods used to study the cell morphology between different studies. In the present study, we have selected clearly visible and tightly packed basal cells within rete pegs at the depth of about 100 μm just before stromal layer for the quantification as these basal cells get blurred or invisible when the microscope scans deeper into the stromal layer. We measured cell size in terms of mean cell area, major and minor diameter, and these results are correlating each other. Moreover, we have also measured shape factors of the basal and intermediate cells by measuring cell solidity and cell compactness. To our knowledge, these shape factors are not reported previously in the literature. From our results, we understand the basal cells in the central cornea are smaller, compact, and more regular in shape in comparison with corneal limbus and scleral limbus. Intermediate cells showed no significant difference in cell size from central to limbus as reported earlier,4, 13 however there was a slight increase in irregularity (cell compactness) of IC from centre towards periphery.
In the present study, we have found no significant differences in cell morphometry between study groups. The effects of contact lens wear on epithelial cells can vary widely based on type, modality, and oxygen transmissibility of the contact lenses. Studies18, 19 have demonstrated that the extended wear hydrogel lenses produce significant alterations on epithelial cell morphology include increased surface cell size and decreased thickness while daily wear soft lenses does not produce any significant effects on epithelial cells. Also it has been reported20, 21 that the short-term wear of high DK extended wear lenses and the habit of eye rubbing produce no alterations in corneal epithelial cell size, regularity, or epithelial thickness. Our contact lens study group wore disposable soft lenses on daily wear basis showed no significant alterations in any of measured morphomeric parameters compared with normal group was consistent to results shown in the literature.
In dry eye syndrome,22 the ocular surface epithelium is compromised due to abnormality in the quality and quantity of the tear film, inflammatory reaction, and altered epithelial permeability and sensitivity. In addition, increased tear osmolity trigger epithelial cell alteration and subsequent epithelial damage. There are IVCM studies23, 24 on dry eyes focussed on quantifying superficial epithelial cells, sub basal nerves and inflammatory cells, but best to our knowledge there are no literature found on quantification of intermediate and basal epithelial cells for comparison. In the present study, the cause of dry eye in group 3 was age-related insufficiency and abnormality in tear film14 and four out of five subjects demonstrated mild-moderate corneal staining (Table 2) on clinical evaluation. Between right and left eyes, the eye with relatively lower signs and symptoms of dryness was selected for cell quantification to acquire more precise cell segmentation and measurements. Although there were some epithelial cellular changes observed clinically correlating with confocal images of dry eye subjects,14 quantitative analysis found no significant microstructural alterations in the intermediate and basal cell layers of the epithelium compared with normal subjects.
From this pilot study, we understand that the observed morphological changes are not significantly affecting the epithelial cell morphometry. However, a further larger study is needed to confirm the results. We believe studying serial images instead of selecting particular images would be more appropriate in providing data on cell areas with morphological changes. In addition, we would consider including larger sample size and further categorizing the subjects based on ocular condition, treatment modality and age in our further larger study.
In conclusion, corneal and limbal epithelial cells could be analysed using IVCM. This study demonstrated quantitative differences in basal and intermediate epithelium between three measured corneal locations (CC, CL, and SL) and found no significant differences in any of the morphometric parameters between contact lens wearers and dry eye subjects compared with normal subjects.

References
Thoft RA, Friend J . The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 1983; 24 (10): 1442–1443.
Tavakoli M, Hossain P, Malik RA . Clinical applications of corneal confocal microscopy. Clin Ophthalmol 2008; 2 (2): 435–445.
Gaujoux T, Touzeau O, Laroche L, Borderie VM . Morphometry of corneal epithelial cells on normal eyes and after anterior lamellar keratoplasty. Cornea 2010; 29 (10): 1118–1124.
Miri A, Al-Aqaba M, Otri AM, Fares U, Said DG, Faraj LA et al. in vivo confocal microscopic features of normal limbus. Br J Ophthalmol 2012; 96 (4): 530–536.
Prakasam RK, Winter K, Schwiede M, Allgeier S, Zhivov A, Köhler B et al. Characteristic quantities of corneal epithelial structures in confocal laser scanning microscopic volume data sets. Cornea 2013; 32 (5): 636–643.
Goldberg MF, Bron AJ . Limbal palisades of Vogt. Trans Am Ophthalmol Soc 1982; 80: 155–171.
Dua HS, Azuara-Blanco A . Limbal stem cells of the corneal epithelium. Surv Ophthalmol 2000; 44 (5): 415–425.
Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A . Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol 2005; 89 (5): 529–532.
Martin R . Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom 2007; 90 (1): 26–30.
Beljan J, Beljan K, Beljan Z . Complications caused by contact lens wearing. Coll Antropol 2013; 37 (Suppl 1): 179–187.
Gobbels M, Spitznas M . Influence of artificial tears on corneal epithelium in dry-eye syndrome. Graefes Arch Clin Exp Ophthalmol 1989; 227 (2): 139–141.
Stave J, Zinser G, Grummer G, Guthoff R . Modified Heidelberg Retinal Tomograph HRT. Initial results of in vivo presentation of corneal structures. Ophthalmologe 2002; 99 (4): 276–280.
Eckard A, Stave J, Guthoff RF . In vivo investigations of the corneal epithelium with the confocal Rostock Laser Scanning Microscope (RLSM). Cornea 2006; 25 (2): 127–131.
Falke K, Prakasam RK, Guthoff RF, Stachs O . In vivo imaging of limbal epithelium and palisades of Vogt. Klin Monbl Augenheilkd 2012; 229 (12): 1185–1190.
Patel DV, Sherwin T, McGhee CN . Laser scanning in vivo confocal microscopy of the normal human corneoscleral limbus. Invest Ophthalmol Vis Sci 2006; 47 (7): 2823–2827.
Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC . Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci 2003; 44 (12): 5125–5129.
Kobayashi A, Sugiyama K . In vivo corneal confocal microscopic findings of palisades of Vogt and its underlying limbal stroma. Cornea 2005; 24 (4): 435–437.
Bourne WM . The effect of long-term contact lens wear on the cells of the cornea. Clao J 2001; 27 (4): 225–230.
Mathers WD, Sachdev MS, Petroll M, Lemp MA . Morphologic effects of contact lens wear on the corneal surface. Clao J. 1992; 18 (1): 49–52.
Kalogeropoulos G, Chang S, Bolton T, Jalbert I . The effects of short-term lens wear and eye rubbing on the corneal epithelium. Eye Contact Lens 2009; 35 (5): 255–259.
Stapleton F, Kasses S, Bolis S, Keay L . Short term wear of high Dk soft contact lenses does not alter corneal epithelial cell size or viability. Br J Ophthalmol 2001; 85 (2): 143–146.
Mantelli F, Massaro-Giordano M, Macchi I, Lambiase A, Binini S . The cellular mechanisms of dry eye: from pathogenesis to treatment. J Cell Physiol 2013; 228 (12): 2253–2256.
Liu Z, Pflugfelder SC . Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology 1999; 106 (5): 939–943.
Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J . An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci 2004; 45 (9): 3030–3035.
Acknowledgements
This work was supported in part by BMBF (RESPONSE) and DFG (KO-4979/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Prakasam, R., Kowtharapu, B., Falke, K. et al. Quantitative assessment of central and limbal epithelium after long-term wear of soft contact lenses and in patients with dry eyes: a pilot study. Eye 30, 979–986 (2016). https://doi.org/10.1038/eye.2016.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.58