Abstract
Purpose
To describe the anterior segment optical coherence tomography (AS-OCT) characteristics of patients with ocular manifestations of mucopolysaccharidoses type I (Hurler), II (Hunter), and VI (Maroteaux–Lamy).
Methods
Prospective, observational study of nine consecutive patients with variants of mucopolysaccharidosis (MPS) attending the Paediatric Ophthalmology service at Manchester Royal Eye Hospital, UK. All patients underwent Visante AS-OCT imaging as part of their ophthalmic assessment.
Results
Ocular involvement tended to be symmetrical. Angle-to-angle distance was significantly lower in MPS VI than in MPS I (P=0.04). Anterior chamber depth, angle opening distance, trabecular-iris space area, and scleral spur angle tended to be lower in MPS VI than in MPS I, but did not reach statistical significance. Corneal thickness in the central 0–2 mm zone was greater in MPS VI than in MPS I, approaching but not attaining statistical significance (P=0.07). The 2–5 and 5–7 mm zones were significantly thicker in MPS VI than MPS I (P=0.04, P=0.04). There was no difference in corneal thickness between MPS I and MPS VI in the peripheral 7–10 mm zone (P=0.57). Measurements of the patient with MPS II resembled the mean values of the MPS I group.
Conclusion
AS-OCT is valuable in quantifying anterior segment pathology in MPS. It suggests more crowded anterior segments and greater corneal thickness in patients with MPS VI than MPS I. AS-OCT is useful in evaluating the risk and mechanism of glaucoma in MPS patients, and may improve our assessment of the efficacy of systemic treatment.
Similar content being viewed by others
Introduction
The mucopolysaccharidoses (MPSs) are a group of progressive, unremitting, multisystem lysosomal storage disorders of which there are several clinical subtypes. The underlying pathophysiology relates to a defect in specific lysosomal enzymes responsible for the catabolism of glycosaminoglycans (GAGs) including dermatan sulphate, heparan sulphate, keratan sulphate, and chondroitin sulphate.1 This leads to accumulation of GAGs within the lysosomes of virtually all tissues of the body, with a diverse range of clinical phenotypes. MPS is hereditary, with inheritance being autosomal recessive apart from MPS II (Hunter) that is X-linked recessive.1 Timely intervention with treatment modalities such as haemopoietic stem cell transplantation and enzyme replacement therapy are known to improve systemic manifestations, quality of life, and survival, but their effect on the ocular complications is less clear.1, 2
With the exception of the relatively recently described MPS IX (Natowicz), ocular abnormalities have been described in all subtypes of MPS. The nature of ocular involvement varies but includes corneal opacification, glaucoma, retinopathy, optic disc swelling, and optic atrophy. However, assessment and monitoring of such complications in patients with MPS is difficult because of age, physical deformity, and, in some cases, learning difficulties hampering cooperation. Glaucoma is particularly difficult to assess because of inaccuracy of intraocular pressure measurement, the presence of corneal clouding precluding assessment of the anterior chamber and angle and visualisation of the optic disc, and the presence of retinopathy influencing visual field assessment.
Anterior segment optical coherence tomography (AS-OCT) is a noncontact, nondestructive imaging modality capable of producing optical cross-sections of the anterior structures of the eye, even in the presence of dense corneal opacity. Resolution is 10–25 times greater than that of ultrasound biomicroscopy,3 and the use of near-infrared light is helpful for imaging photophobic patients. The rapid acquisition time is beneficial in the context of uncooperative patients, with neonates as young as a few days having been successfully imaged.3 AS-OCT has transformed the way in which ophthalmologists can assess the eye in a multitude of conditions. In addition to being of clinical value, demonstration of pathology can be very helpful with patient and carer education and may improve treatment compliance.4 AS-OCT provides an invaluable tool for assessment and monitoring of the ocular complications in MPS. This article provides, to our knowledge, the first description of the AS-OCT findings in patients with MPS I, II, and VI.
Materials and methods
Ethical approval was obtained (ethics number: 10/H1013/16 (30 April 2010)). Informed consent was obtained and the guidelines of the Declaration of Helsinki (1995) were adhered to throughout.
Nine consecutive patients with known MPS were prospectively recruited from the paediatric ophthalmology department at Manchester Royal Eye Hospital (MREH). Each patient underwent AS-OCT with the Visante system (version 2.0.1.88; Carl Zeiss Meditec AG, Jena, Germany). Images were obtained in the meridian of 180°–0°, thus analysing the temporal and nasal angles. This formed part of the routine assessment that also included measurement of intraocular pressure with either Goldmann applanation (HAAG-STREIT International, Koeniz, Switzerland) or iCare rebound tonometry (Icare Finland Oy, Vantaa, Finland).
Using images acquired from AS-OCT, central corneal thickness (CCT; μm) was measured. This was in addition to more detailed global pachymetric analysis of the cornea in four zones: the central 0–2 mm zone, the middle 2–5 mm and 5–7 mm zones, and the peripheral 7–10 mm zone. In order to analyse the anterior chamber depth (ACD), CCT was subtracted from the measured ACD to give the true ACD (mm).
Angle opening distance (mm) and trabecular-iris space area (TISA, mm2) at both 500 and 750 μm from the scleral spur were also determined (AOD500, AOD750, TISA500, TISA750). AOD is the perpendicular distance from anterior iris to the trabecular meshwork, and is measured at both 500 and 750 μm anterior to the scleral spur. TISA is the area enclosed by the AOD respectively, the corneoscleral wall, the anterior iris, and a perpendicular line drawn between the scleral spur and iris.
Other data obtained from Visante AS-OCT included angle-to-angle distance (ATA; mm), crystalline lens rise (CLR; μm), and scleral spur angle (SSA; °). ATA is calculated as the distance between the two iridocorneal angles (ie, nasal and temporal). CLR is the perpendicular distance from the straight ATA line and the anterior lens surface. SSA is a measurement of the angle formed at the apex of the iris recess, with the arms of the angle passing through the AOD500 line.
Statistical analysis to compare the MPS I and MPS VI groups was undertaken with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) using the nonparametric Mann–Whitney U-test. Correlation between dependent variables was analysed using Pearson’s linear coefficient. Statistical analysis of the MPS II group was not undertaken because of there being only one patient in this group.
Results
Five patients had MPS I (Hurler), one patient had MPS II (Hunter), and three patients had MPS VI (Maroteaux–Lamy) (see Tables 1 and 2). All patients had relatively symmetrical anterior segment involvement between each eye, and all except the patient with MPS II had corneal clouding (Table 1). The measurements for the right eye of each patient were selected for analysis, with the temporal angle (180°) used for angle-specific measurements (AOD, TISA, and SSA; Table 2).
MPS I (Hurler)
Anterior segment measurements
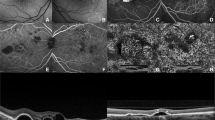
Patients 3, 4, 5, 7, and 8 had a diagnosis of MPS I (n=5) and all had mild or moderate corneal clouding (Figures 1a–d and 2). CCT (mean±SD) was 560±84 μm, and ACD was 2.58±0.62 mm. Mean ATA was 12.55±0.34 mm. Mean CLR was 449±508 μm.
Angle-specific measurements (180°)
Mean AOD500 and AOD750 were 0.535±0.361 and 0.727±0.459 mm, respectively. Mean TISA500 and TISA750 were 0.179±0.122 and 0.341±0.224 mm2, respectively. Mean SSA was 37.3±27.0°.
Global pachymetry
Mean corneal thickness in the central 0–2 mm zone was 573±83 μm. This increased to 713±104 μm in the peripheral 7–10 mm zone, a mean difference of 139±27 μm.
MPS II (Hunter)
Anterior segment measurements
Patient 1 was a 5.9-year-old male with MPS II and clinically clear corneae (Figures 1e and f). Regarding the right eye, CCT was 510 μm and ACD was 2.30 μm, lower values than for the MPS I group. ATA was 12.24 mm and CLR was 380 μm.
Angle-specific measurements (180°)
AOD500 and AOD750 were 0.548 and 0.700 mm, respectively. TISA500 and TISA750 were 0.179 and 0.342 mm2, respectively. SSA was 48.1°.
Global pachymetry
Corneal thickness in the central 0–2 mm zone was 508 μm. This increased to 584 μm in the peripheral 7–10 mm zone, a difference of 76 μm.
MPS VI (Maroteaux–Lamy)
Anterior segment measurements
Patients 2, 6, and 9 had a diagnosis of MPS VI (n=3) and marked corneal clouding, with an age (mean±SD) of 16.5±1.2 years, thus representing an older group (Figures 1g and h). Mean CCT was 790±277 μm, and mean ACD was 2.05±0.27 mm. Mean ATA was 11.34±0.62 mm. Mean CLR was 737±257 μm.
Angle-specific measurements (180°)
Mean AOD500 and AOD750 were 0.20±0.09 and 0.24±0.13 mm, respectively. Mean TISA500 and TISA750 were 0.09±0.02 and 0.14±0.04 mm2, respectively. Mean SSA was 20.5±8.5°.
Global pachymetry
Mean corneal thickness in the central 0–2 mm zone was 812±183 μm. Mean corneal thickness in the peripheral 7–10 mm zone was 842±209 μm. It should be noted that in two patients, corneal thickness increased towards the periphery, as occurs in the normal eye. However, in one patient with significant central corneal thickening, the corneal thickness actually decreased peripherally by a difference of 417 μm.
Comparison between MPS I and MPS VI
The right eyes of the MPS I and MPS VI groups (n=8) were compared using the nonparametric Mann–Whitney U-test (15 pairings). Because of low statistical power secondary to small sample sizes, only very large effects (U(8)=0 or U(8)=15) were considered significant (P<0.05). Because of the potential for type II error (false negative), we have reported the effect size, r, in addition to the P-value.
The two groups differed in age, with the MPS VI group tending to be older than the MPS I group. However, although this difference was large, it did not quite reach statistical significance (U(8)=14, Z=1.94, P=0.07, effect size r=−0.69).
ATA was significantly lower in MPS VI than in MPS I, with a large effect size (U(8)=0, Z=−2.24, P=0.036, effect size r=−0.79). ACD, AOD500, AOD750, TISA500, and TISA750 also tended to be lower in MPS VI than in MPS I, with moderately large effect sizes (r=−0.47 for all five parameters). However, these were not statistically significant (U(8)=3, Z=−1.34, P=0.25). No statistically significant difference in CLR (U(8)=11, P=0.39) or SSA (U=3, P=0.40) was found between the two groups, although there was a tendency for CLR to be greater and SSA smaller in MPS VI than in MPS I (medium effect sizes, r=0.37 and r=−0.38, respectively). There was wide variation in CLR in MPS I, with values both higher and lower than those in MPS VI, in which measurements were more uniform.
CCT tended to be greater in MPS VI than in MPS I, but although the effect size was large (r=0.58), this did not attain statistical significance (U(8)=13, Z=1.64, P=0.14). A similar but more significant result was obtained via global pachymetry, with the central 0–2 mm zone being thicker in MPS VI than in MPS I (U(8)=14, Z=1.94, P=0.07, effect size r=0.69). The 2–5 mm zone was significantly thicker in MPS VI than MPS I (U(8)=15, Z=2.25, P=0.04, effect size r=0.80), as was the 5–7 mm zone (U(8)=15, Z=2.24, P=0.04, effect size r=0.79). There was no statistically significant difference between MPS I and MPS VI in the peripheral 7–10 mm zone (U(8)=10, Z=0.75, P=0.57).
Discussion
Anterior chamber and angle morphology
The amount of published data in terms of AS-OCT normal values in adults is increasing, but there is a distinct lack of such normal data for children. One of the earliest studies of AS-OCT, with measurements made manually using computer callipers, found a mean anterior chamber width of 12.53±0.47 mm, mean ACD of 2.99±0.323 mm, and mean CLR of 0.39±0.27 mm.5 A prospective study of 497 normal, phakic eyes of adults aged >50 years compared Visante AS-OCT, IOLMaster (Carl Zeiss Meditec AG), and scanning peripheral anterior chamber depth analyser (SPAC, Takagi, Japan) measurements, and the AS-OCT mean ACD was 3.14 mm.6 A further AS-OCT study of 1069 normal eyes in Singaporean adults (mean age 56.9±9.5 years) found mean ACD to be 3.29±0.35 mm.7 A Canadian study of 40 normal, adult eyes found the mean nasal angle to be 39.32±3.07°.8 Work conducted in Turkey on 40 normal eyes, using Visante OCT, found the mean nasal and temporal angles to be 31.2±8.7° and 32.1±8.5°, respectively.9 Finally, a study comparing AS-OCT with slit-lamp OCT in 49 normal, Chinese eyes found a mean temporal AOD500 of 572±275 μm, mean temporal TISA500 of 0.193±0.102mm2, and mean temporal trabecular-iris angle (TIA, roughly equivalent to SSA) of 39.6±13.2°.10 This study also found the mean nasal angle metrics to be narrower than those of the temporal angle.10
Although it is likely that age will affect the parameters, some interesting comparisons can still be made between these normal adult values and our study of children with MPS. For example, the mean ATA values in MPS I (12.55 mm) and MPS II (12.53 mm) were very similar to the mean anterior chamber width found by Goldsmith et al,5 whereas in MPS VI the value was significantly smaller (11.34 mm). Similarly, previously published values for mean ACD5, 6, 7 are much greater than those in our MPS VI patients, but only slightly greater than in MPS I and II. The mean CLR found by Goldsmith et al5 was roughly equivalent to the mean values in MPS I and II (449 and 380 μm, respectively), but much lower than in MPS VI (737 μm). Regarding the angle itself, Leung et al10 found a considerably greater mean AOD500 and TISA500 in their study than we found in MPS VI, but their values were almost equivalent to our MPS I and MPS II patients.
Table 1 shows that most of our cohort were hypermetropic. The influence of refractive error on Visante AS-OCT values has previously been investigated in otherwise healthy subjects.11 As one would expect, hypermetropia resulted in narrower angles than in myopia, with the mean AOD500 in normal hypermetropic subjects being 0.372 mm and the mean TISA500 being 0.131 mm2.11 The angle parameters found in these normal hypermetropic subjects were considerably less compact than those found in our MPS VI patients, but similar or more compact than the patients with MPS I or II.
As a general rule, each patient in our study had relatively symmetrical involvement between each eye. One exception was patient 2 (MPS VI), who had a considerably greater CLR in his left eye (800 μm) than in his right eye (440 μm). This corresponded to a substantially narrower angle in the left eye in terms of ACD, SSA, AOD, and TISA, including full closure of the nasal angle.
In contrast to the other four patients with MPS I and fairly open angles, patient 7, a 6-year-old female, had bilateral angle closure (SSA 0°) (Figures 1c and d). The AOD500 and 750 and the TISA500 and 750 equalled 0 mm and 0 mm2 bilaterally at both 0° and 180°. Central corneal thickness was normal (560 μm OD, 530 μm OS) and peripheral corneal thickness was similar to the other patients with MPS I. ATA was similar to the other MPS I patients and greater than the MPS VI group (12.33 mm OD, 12.18 mm OS). However, her ACD was the lowest (1.61 mm OD, 1.56 mm OS) and her CLR the highest (1040 μm OD, 970 μm OS) of all the patients studied. This patient developed raised IOP and glaucomatous optic neuropathy.
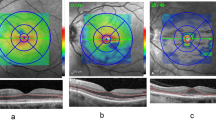
Raised CLR (or lens vault) and low ACD are known to be risk factors for the development of primary angle closure (PAC).12, 13 Chen et al14 found significant correlation between decreasing ACD and increasing risk of PAC (odds ratio=3.59 for 0.2 mm decrease in ACD). Therefore, attention should be paid to CLR and ACD, in addition to the parameters specific to the angle itself when monitoring patients with MPS. Figures 2a–c shows data for the right eyes from all nine patients in our study, revealing strong negative correlation between CLR and ACD (r=−0.83, Pearson’s linear coefficient), moderate negative correlation between CLR and AOD500 (r=−0.52), and, correspondingly, positive correlation between ACD and AOD500 (r=0.70).
Corneal thickness
It is well known that differences in CCT affect IOP estimation in the normal population. Corneal hysteresis has also been shown to affect IOP readings in normal patients and those with MPS,15 although this was not investigated in our study. CCT in healthy children is known to increase up to the age of 11 years, after which it tends to plateau at ∼573 μm in white and Hispanic and 551 μm in African-American children.16 The majority of the MPS I group in our cohort had CCT comparable with or slightly below the CCT of the normal paediatric population, with a similar conclusion having been reached by other authors.17 The exception to this was patient 4 who was considerably older (16.2 years) than the rest of the MPS I group (mean 7.44 years). This patient’s CCT was increased (680 μm) as compared with the other MPS I patients, which may correspond with the natural history of the disease process. However, it should be noted that this theory is only supported by a single patient, and therefore warrants further investigation. The oldest patient in the MPS VI group (patient 9, Figures 1g and h) also demonstrated a substantially greater CCT than the two younger patients with the same condition, although the age differences were only 1.3 and 2.4 years. In this case, it is perhaps less likely to represent the natural disease course, as such a change would be surprising in such a short period. Interestingly, a previous study of CCT in MPS found no significant correlation between CCT and age, despite MPS being progressive in nature.17
The same study demonstrated moderate correlation between CCT and the degree of corneal opacification (r=0.57, Pearson’s linear correlation).17 We subjectively graded corneal opacification as none, mild, moderate, or severe, and found a similar association with CCT. Only the patient with MPS II was graded as having no corneal opacification (mean CCT 515 μm). One patient with MPS I had mild opacification (mean CCT 495 μm). Six patients with MPS I and MPS VI had moderate opacification (mean CCT 587 μm). One patient with MPS VI had severe opacification (mean CCT 1175 μm).
In contrast to our study, where there was a trend for increased CCT compared with the normal range in patients with MPS VI, a previous study of nine patients with MPS VI found median CCT to be 547 μm (range 492.5–693.05 μm), as measured ultrasonographically.18
With one exception, global pachymetry in our study demonstrated an increase in corneal thickness towards the periphery, as in the normal cornea. Prospero Ponce et al19 compared Visante AS-OCT with various imaging modalities in 103 normal subjects and patients with other corneal pathologies. A peripheral corneal thickness of 515.4±29.2 μm was found in their normal-subject group, which is well below the peripheral corneal thickness values we found in patients with MPS I and VI.
Differences between central and peripheral corneal thickness varied between our groups, with the right eyes of the MPS I group increasing by a mean of 139±27 μm towards the periphery. The patient with MPS II had a smaller increase in thickness of 76 μm OD, 73 μm OS towards the corneal periphery. In the MPS VI group, the corneal thickness of patient 9 decreased towards the periphery by −417 μm OD, −131 μm OS, whereas in the other two patients it increased by a mean 255 μm (range 201–308 μm).
Summary
Overall, our Visante AS-OCT findings suggest a narrower and more compact anterior segment and angle configuration in MPS VI than in MPS I. It should be noted that because of the small sample size, only very large effects will be statistically significant. However, all the effect sizes generated in our analysis were medium to large, indicating that the dependent variables are all worthy of further study with greater numbers of patients.
The difference in age between the two groups, although not statistically significant, is an acknowledged limitation of the data. Whether our results reflect true differences between these two MPS subtypes, or just a natural progression of disease, is a question that can only be properly addressed by a larger cohort with age-matched control groups. However, although the one older patient with MPS I (aged 16.2 years) had an increased corneal thickness, he had a similar angle configuration and indeed a greater ACD compared with the other MPS I patients. This adds substance to the theory that MPS I is characterised by a more open anterior segment than is found in MPS VI, despite age differences.
Our data also show that both MPS I and MPS VI patients tend to have greater central and peripheral corneal thickness than the one patient with MPS II. This appears to correlate with the fact that patients with MPS I and VI tend to have corneal clouding, whereas in MPS II the cornea is usually clinically clear. A larger cohort of MPS II patients is required to substantiate this finding.
It is clear that the detailed, objective information regarding corneal thickness, ACD, CLR, and angle morphology provided by AS-OCT is of great importance in MPS, particularly in the context of glaucoma evaluation and management, given the inherent difficulties in assessing these patients. It is also likely to have other applications in terms of assessing the efficacy of nonsurgical treatment and in surgical planning.
Evolving treatment modalities, including bone marrow and stem cell transplantation (BMT and SCT) and enzyme replacement therapy (ERT), have been shown to benefit patients with MPS in terms of both life expectancy and systemic health.1, 20, 21, 22, 23, 24 However, debate remains surrounding the effect of these treatments on the ocular manifestations, although animal studies have demonstrated prevention and/or clearance of corneal stromal GAGs accumulation following early treatment with ERT.25 To date, evaluation of ocular response to treatment has depended on slit-lamp assessment, ultrasound, and electrodiagnostics.2, 3 AS-OCT is likely to improve this.
Surrogate markers such as visual acuity or patient-reported symptoms may not always be reliable in patients with MPS. AS-OCT will facilitate detailed, regular, and noninvasive monitoring of patients undergoing treatment in order to ascertain stabilisation or deterioration in terms of anterior segment characteristics.
AS-OCT will also be helpful in improving preoperative planning. Corneal graft surgery, increasingly in the form of deep anterior lamellar keratoplasty (DALK), can provide good results in MPS patients, provided visual potential is not compromised by coexistent posterior segment involvement. UBM has previously been used to guide trephination depth,26 but AS-OCT may provide additional benefits in this domain, as well as improving assessment of graft integrity postoperatively.
Conclusion
This study is the first to describe AS-OCT characteristics in patients with MPS, and demonstrates interesting differences between those MPS subtypes studied. Despite the limitations of small numbers and age differences, useful observations have still been made regarding the different subtypes of MPS within our cohort, with further reference to published data.
The advent of AS-OCT promises to increase our knowledge of anterior segment geometry and corneal thickness in MPS, improving our ability to correlate these with other variables such as IOP and corneal clarity. The finding of peripheral corneal thickening on detailed pachymetry may help elucidate the mechanism of raised IOP in patients with MPS I and VI. Other metrics such as ACD, ATA, and CLR are also likely to prove valuable in the routine assessment of such patients. Large-scale studies to address the current lack of normal AS-OCT data in healthy children would be greatly beneficial—not just in the context of MPS but also in the evaluation of other conditions with anterior segment manifestations.
Further work comparing a larger cohort of MPS patients with age-matched controls, and undertaking AS-OCT studies on other subtypes of MPS would add to our understanding. This is likely to require a multicentre approach because of the rarity of these conditions. In the meantime, the authors recommend the use of AS-OCT routinely in the initial evaluation and subsequent follow-up of patients with MPS.

References
Ashworth JL, Biswas S, Wraith E, Lloyd IC . Mucopolysaccharidoses and the eye. Surv Ophthalmol 2006; 51 (1): 1–17.
Ferrari S, Ponzin D, Ashworth JL, Fahnehjelm KT, Summers CG, Harmatz PR et al. Diagnosis and management of ophthalmological features in patients with mucopolysaccharidosis. Br J Ophthalmol 2011; 95 (5): 613–619.
Majander AS, Lindahl PM, Vasara LK, Krootila K . Anterior segment optical coherence tomography in congenital corneal opacities. Ophthalmology 2012; 119: 2450–2457.
Mireskandari M, Tehrani NN, VandenHoven C, Ali A . Anterior segment imaging in pediatric ophthalmology. J Cataract Refract Surg 2011; 37: 2201–2210.
Goldsmith JA, Li Y, Chalita MR, Westphal V, Patil CA, Rollins AM et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology 2005; 112 (2): 238–244.
Lavanya R, Teo L, Friedman DS, Aung HT, Baskaran M, Gao H et al. Comparison of anterior chamber depth measurements using the IOLMaster, scanning peripheral anterior chamber depth analyser, and anterior segment optical coherence tomography. Br J Ophthalmol 2007; 91 (8): 1023–1026.
Ang M, Chong W, Huang H, Tay WT, Wong TY, He MG et al. Comparison of anterior segment optical tomography parameters using a semi-automatic software to standard clinical instruments. PLoS One 2013; 8 (6): e65559.
Sorbara L, Maram J, Fonn D, Woods C, Simpson T . Metrics of the normal cornea: anterior segment imaging with the Visante OCT. Clin Exp Optom 2010; 93 (3): 150–156.
Dinc UA, Oncel B, Gorgun E, Yalvac IS . Assessment of anterior chamber angle using Visante OCT, slit-lamp OCT, and Pentacam. Eur J Ophthalmol 2010; 20 (3): 531–537.
Leung CK, Li H, Weinreb RN, Liu J, Cheung CY, Lai RY et al. Anterior chamber angle measurement with anterior segment optical coherence tomography: a comparison between slit lamp OCT and Visante OCT. Invest Ophthalmol Vis Sci 2008; 49 (8): 3469–3474.
De Orta-Arellano F, Muñoz-Rodriguez P, Salinas-Gallegos JL . Measurement of anterior chamber angle with optical coherence tomography. In: Kubena T, Kofronova M, (eds) The Mystery of Glaucoma. InTech: Rijeka, Croatia, 2011 pp 221–227.
Guzman CP, Gong T, Nongpiur ME, Perera SA, How AC et al. Anterior segment optical coherence tomography parameters in subtypes of primary angle closure. Invest Ophthalmol Vis Sci 2013; 54 (8): 5281–5286.
Razeghinejad MR, Banifatemi M . Ocular biometry in angle closure. J Ophthalmic Vis Res 2013; 8 (1): 17–24.
Chen YY, Chen YY, Sheu SJ, Chou P . The biometric study in different stages of primary angle-closure glaucoma. Eye 2013; 27 (9): 1070–1076.
Fahnehjelm KT, Chen E, Winiarski J . Corneal hysteresis in mucopolysaccharidosis I and VI. Acta Ophthalmol 2012; 90 (5): 445–448.
Pediatric Eye Disease Investigator Group. Central corneal thickness in children. Arch Ophthalmol 2011; 129 (9): 1132–1138.
Connell P, McCreery K, Doyle A, Darcy F, O’Meara A, Brosnahan D . Central corneal thickness and its relationship to intraocular pressure in mucopolysaccararidoses-1 following bone marrow transplantation. J AAPOS 2008; 12 (1): 7–10.
Kottler U, Demir D, Schmidtmann I, Beck M, Pitz S . Central corneal thickness in mucopolysaccharidosis II and VI. Cornea 2010; 29 (3): 260–262.
Prospero Ponce CM, Rocha KM, Smith SD, Krueger RR . Central and peripheral corneal thickness measured with optical coherence tomography, Scheimpflug imaging, and ultrasound pachymetry in normal, keratoconus-suspect, and post-laser in situ keratomileusis eyes. J Cataract Refract Surg 2009; 35 (6): 1055–1062.
Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children The Storage Disease Collaborative Study Group. Blood 1998; 91 (7): 2601–2608.
Wraith JE . Lysosomal disorders. Semin Neonatal 2002; 7 (1): 75–83.
Summers CG, Purple RL, Krivit W, Pineda R 2nd, Copland GT et al. Ocular changes in the mucopolysaccharidoses after bone marrow transplantation. A preliminary report. Ophthalmology 1989; 96 (7): 977–984 discussion 984–985.
Gullingsrud EO, Krivit W, Summers CG . Ocular abnormalities in the mucopolysaccharidoses after bone marrow transplantation. Longer follow-up. Ophthalmology 1998; 105 (6): 1099–1105.
Vellodi A, Young EP, Cooper JE, Wraith JE, Winchester B, Meaney C et al. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child 1997; 76: 92–99.
Newkirk KM, Atkins RM, Dickson PI, Rohrbach BW, McEntee MF . Ocular lesions in canine mucopolysaccharidosis I and response to enzyme replacement therapy. Invest Ophthalmol Vis Sci 2011; 52 (8): 5130–5135.
Harding SA, Nischal KK, Upponi-Patil A, Fowler DJ . Indications and outcomes of deep anterior lamellar keratoplasty in children. Ophthalmology 2010; 117 (11): 2191–2195.
Acknowledgements
This research was facilitated by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JLA has received an educational grant from Biomarin Europe Ltd. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahmed, T., Turnbull, A., Attridge, N. et al. Anterior segment OCT imaging in mucopolysaccharidoses type I, II, and VI. Eye 28, 327–336 (2014). https://doi.org/10.1038/eye.2013.281
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.281
Keywords
This article is cited by
-
Glaucoma in mucopolysaccharidoses
Orphanet Journal of Rare Diseases (2021)
-
Multimodal imaging of Hurler syndrome-related keratopathy treated with deep anterior lamellar keratoplasty
BMC Ophthalmology (2020)