Abstract
Background
Necrotising fasciitis (NF) is a devastating disease with considerable mortality and morbidity, and early aggressive surgical debridement of devitalised necrotic tissues has traditionally been advocated.
Methods
We describe three patients who were referred from other units several weeks after developing periocular necrotising fasciitis; in all the three, the disease had been managed medically without surgical debridement, with apparent ‘stalling’ of the inflammatory process despite persistent necrotic periocular tissue.
Results
Following ‘elective debridement’ of the devitalised tissues and reconstruction with local flaps, all achieved a satisfactory aesthetic result.
Discussion
The role of host genetic determinants, polarised cytokine responses, and early, effective medical treatment in patients with atypical ‘disease phenotypes’ in NF are discussed.
Similar content being viewed by others
Introduction
Necrotising fasciitis (NF) is a destructive microbial infection of the subcutaneous tissue and skin, with a ‘toxic interface’ between healthy and diseased tissue that can progress rapidly, and cause major regional and systemic morbidity or death.1 NF is most common in the abdominal wall, perineum and extremities, with the least affected area being the cervico-facial region (<10% of all cases);2 periorbital involvement is rare,3 with associated mortality (14.4%) being markedly lower than for the disease elsewhere.3 The most common causative bacteria have been grouped into type 1 (polymicrobial infections, consisting of a mixture of anaerobes, Gram-negative bacilli and enterococci) and type 2 (group A β-haemolytic Streptococcus with or without associated staphylococcal infection).
Clinical features of periorbital NF can include severe pain, swelling, fever, haemorrhagic bullae, skin necrosis and, with bacteraemia, rapid progression to systemic organ failure, and death.4 Although patients who develop periorbital NF tend not to be immunocompromised, frequently there is a history of minor skin trauma, an insect bite, or surgery (such as blepharoplasty).
The natural course of periorbital NF differs significantly to disease elsewhere, this attributed to the rich blood supply of the periocular tissues;5, 6 in addition, facial involvement may be more immediately apparent, and therefore treated earlier. Although empirical antibiotic treatment and early, aggressive surgical debridement of necrotic infected tissue is widely advocated (and frequently necessary), in the absence of systemic involvement a more conservative medical approach with delayed surgical debridement can be considered.7
We present three cases of periocular NF in which the disease appeared to have ‘stalled’ following medical treatment alone; in these patients, persistent devitalised periocular tissue remained adherent to normal healthy structures, but without an inflamed ‘toxic interface’ and the progression of disease so characteristic of NF.
Case Reports
Case 1
A previously fit 68-year-old Caucasian man was referred to the Adnexal Department with bilateral periocular NF. He had a history of hypertension, for which he was taking amlodipine 10 mg daily, and a mild polysensory neuropathy for which he took dexketoprofen 25 mg thrice daily, and clonazepam. He could not recall any preceding eyelid trauma, smoked 60 cigarettes a day, and consumed 40 units of alcohol per week.
Eight weeks prior to the presentation, he had developed mild gastroenteritis with associated periocular swelling and redness, and diagnosed with conjunctivitis, was prescribed topical ocular antibiosis. While abroad a week later, he became acutely unwell with Pseudomonas septicaemia and secondary renal failure, and was admitted to the local intensive care unit, where he remained for 2 weeks. With bilateral periocular swelling and dark-purple discolouration of the skin across the nasal bridge, periocular skin swabs were positive for Pseudomonas aeruginosa, and he was managed with intravenous ciprofloxacin alone.
Immediately on his return from abroad (this being 6 weeks after developing periocular NF), he presented to our unit with severe bilateral periocular exposure and a visual acuity of hand movements in each eye. The periocular regions were distorted with eschar formation, severe cicatricial ectropion and lid retraction, lagophthalmos, and chemosis (Figure 1a). Despite such marked changes, the adjacent vitalised tissues were not inflamed, the only ‘active’ disease process being bilateral Candida albicans keratitis with hypopyon, this promptly responding to treatment with topical amphotericin B.
(Case 1) (a) Clinical photograph of eschar formation over the lateral aspect of the right eye, and bilateral cicatricial ectropion with chemosis and lagophthalmos. (b) There is a clear interface between the mummified necrotic tissue (this being removed en bloc) and healthy, well-vascularised adjacent tissues—with remarkable preservation of pretarsal eyelid skin. (c) Following full periocular reconstruction, ocular protection is improved with the visual acuity rising to 6/9 in each eye.
In view of the marked ocular surface exposure and inflammatory changes, the patient underwent immediate debridement, complete release of the retractor complexes in all four eyelids, and limited tarsorrhaphies under general anesthesia. Peroperatively, a clear interface was found between the mummified necrotic tissue and entirely healthy, well-vascularised adjacent tissues, with no active inflammatory changes and remarkable preservation of pretarsal eyelid skin (Figure 1b). Periocular reconstruction entailed a two-stage vascular pedicle flap to the right upper lid and periorbital skin grafting to the upper and lower eyelids, with later lacrimal drainage surgery (with insertion of glass lacrimal bypass tubes). With improved corneal protection (Figure 1c), the visual acuity gradually improved to 6/9 in each eye, although, with relatively static eyelids, some lacrimal symptoms persist.
Case 2
Three weeks after a minor eyelid laceration, a 57-year-old man rapidly developed marked erythema, swelling, and tenderness of the periorbital region. Apart from suffering from osteoarthritis, for which he took ibuprofen, he was otherwise well. His family practitioner had prescribed a week’s course of flucloxacillin (250 mg four-times daily) shortly after onset, with a marked improvement in the inflammatory signs and symptoms. Five days after injury, the left upper eyelid skin became dusky and mottled, and began to weep ‘serum’ from areas of epithelial breakdown. A week later, the central area of necrosis was contracting and the gaps around the area of necrosis increased in size. A skin swab had grown group A β-haemolytic Streptococcus.
The patient presented to the ophthalmic casualty 3 weeks after onset of symptoms, and examination revealed minimal inflammation in the healthy tissues around a large area of ‘mummifying’ necrotic tissue in the left upper eyelid (Figure 2a). After 3 days of high-dose intravenous benzyl penicillin and cefuroxime, the necrotic upper lid tissues were debrided to allow development of healthy granulation tissue (Figure 2b). The final result was satisfactory after secondary upper eyelid repair with skin grafting, with adequate eyelid closure and ocular protection (Figure 2c).
Case 3
A 76-year-old woman presented with a 3 week history of a red and tender right upper eyelid, following a minor scratch. She had been well, although suffered with chronic rheumatoid arthritis controlled with diclofenac. A partial response was achieved with a 5-day course of low-dose oral antibiotics (ampicillin/flucloxacillin; 250 mg/250 mg four-times daily), but within a week the eyelid skin became dusky and then yellow, developing areas of breakdown around the edges of the inflamed tissues. After 3 weeks from the initial onset of symptoms, she presented to the Adnexal Department with ‘mummified’ necrotic periorbital soft tissue and a picture of ‘stalled’ disease very similar to that described above (Figure 3a). Limited debridement of these devitalised structures (Figure 3b) revealed clear tissue planes between viable and non-viable tissues. Following full-thickness skin grafting to the upper lid, the patient achieved a very acceptable functional and aesthetic result (Figure 3c).
Discussion
NF is a clinical diagnosis and can occur without a prior history of injury or immunocompromise.3 Prompt antibiosis can reduce not only local tissue destruction—mediated by acute-phase cellular infiltration and release of lytic enzymes—but also the risk of bacteraemia and systemic organ failure. Aggressive surgical debridement can also prevent the subcutaneous extension of bacterial infection and accompanying tissue lysis.2 Although widespread surgical debridement is clearly indicated where there is uncontrolled disease, in certain individuals the inflammatory/infective interface can be contained by antibiosis alone, following which the active inflammatory process wanes, and the (presumed) sterile and devitalised tissues persists along-side healthy tissues without a pathogenic effect. This is similar to the situation where an avascular and necrotic full-thickness skin graft remains as a devitalised tissue alongside healthy tissues, but without inflammation or infection of the neighbouring structures.
The three patients were referred from other units after some time, and clearly demonstrate that, despite quite extensive periocular involvement, debridement is not always essential. This suggests that the inflammatory and lytic phase of NF can—in some cases—be aborted with antibiotics alone, with debridement only being required at a later stage to permit reconstruction. Although we would continue to recommend wide debridement where there is progressive disease and/or evidence of bacteraemia or systemic toxicity, it should be remembered that in some patients the disease will ‘stall’ on medical treatment alone, and that close observation with appropriate antibiosis may suffice.
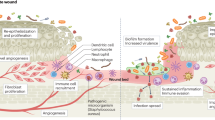
The reasons for this aborted pattern of disease—in contrast to the life-threatening picture seen in some healthy individuals—remain unclear. Earlier recognition of serious disease and prompt institution of more effective antibiosis probably limits the ‘window’ for early bacterial proliferation and production of exotoxins. This, in turn, would limit extravasation of pro-inflammatory Th1 cells into the affected tissues, with a consequent reduction in the accrual of cytotoxic and lytic ‘Th1’ pro-inflammatory cytokines (these including Interleukin-2 (IL-2), IL-6, TNF-α, TNF-β (Leukotriene), and Interferon γ), with containment of the early drive to inflammation and a 'mopping up' of the causative pathogen(s).
In addition to early disease recognition and treatment, host genetic factors could also play a role. In Streptococcal infections, superantigens (SAgs) form a molecular bridge between certain HLA-II molecules on antigen-presenting cells (APCs) and T cell receptors on T cells, thus resulting in strong activation of both cell types and the production of pro-inflammatory cytokines.8 But the extent of the human cellular and cytokine immune response to microbial challenge is genetically determined, varies considerably between one patient and another, and is independent of the virulence of the infecting bacterial strain.9, 10, 11 Individuals whose genome contains fewer pro-inflammatory genetic polymorphisms mount an abrogated response to microbiological challenge, with associations between proinflammatory gene polymorphisms and clinical outcomes recorded for a wide variety of infections, including streptococcal septic shock,11, 12, 13, 14, 15 community acquired pneumonia,16 and meningococcal meningitis17 (Table 1).
These associations support a role for host pro-inflammatory genes in determining both the strength of the immune response and the clinical outcome in streptococcal infections. Specific human leucocyte antigen (HLA) haplotypes are associated with an amplified inflammatory response and consequently a worse outlook for severe systemic disease. This is thought to result from the ability of Streptococcal antigens to bridge between certain HLA types and T cells, with a polarisation towards a pro-inflammatory Th1 inflammatory response. Thus, in patients with the pro-inflammatory HLA-II-DR14/DR7/DQ5 haplotype, T cell activation and the production of pro-inflammatory cytokines is relatively increased (with a simultaneous lower production of the anti-inflammatory IL-10 molecule), this significantly increasing the risk of developing toxic shock syndrome. In contrast, individuals with the 'protective' HLA-II-DR15/DQ6 haplotype have a lower risk of severe sepsis.18 These observations in humans are supported by studies which show that transgenic mice expressing only the protective HLA-II DQ6 allele are also protected from severe sepsis when infected with Group A Streptococcus.19 Whether either of these HLA-II alleles exert a direct influence on cytokine production (via their presentation of Stretococcal superantigen to antigen presenting cells), or is in linkage disequilibrium with other pro-inflammatory gene polymorphisms in the MHC, remains unknown.
A final consideration is the epidemiology of streptococcal infections. Invasive infections (eg, erysipelas and scarlet fever) were relatively infrequent between the 1920s and 1980s, with severe cases of streptococcal toxic shock syndrome and NF increasing dramatically thereafter.20 At the same time, certain persistent and hypervirulent strains emerged, these including a subclone of the M1T1 serotype.21 However, in our experience, the incidence of severe adult periocular NF with systemic toxicity—while high in the 1990s and early 2000s—appears to have reduced over the past decade. Although probably due to increased awareness of the dangers of this soft tissue infection (and consequent earlier treatment), this observation might also signal a shift from hypervirulent to less virulent Streptococcal strains in recent years.
In summary, the patients described in this report presented with periocular NF that was contained by intravenous or oral antibiosis. In the first patient, the septicaemia preceded the periocular NF, and neither of the other two developed systemic disease. None underwent surgical debridement, and in all, the diseases appeared to ‘stall’, with necrotic tissues remaining adherent to the healthy vascularised tissues, and without a clear toxic interface, for as long as 6 weeks. To our knowledge, this is the first report of such marked and sustained ‘stalling’ of a disease, which can often have disastrous consequences even with aggressive surgical debridement.

References
Bisno AL, Stevens DL . Streptococcal infections of skin and soft tissues. N Engl J Med 1996; 334: 240–245.
Ord R, Coletti D . Cervico-facial necrotising fasciitis. Oral Dis 2009; 15: 133–141.
Lazzeri D, Lazzeri S, Figus M, Tascini C, Bocci G, Colizzi L, Giannotti G, Lorenzetti F, Gandini D, Danesi R, Menichetti F, Del Tacca M, Nardi M, Pantolini M . Periorbital necrotising fasciitis. Br J Ophthalmol 2010; 94: 1577–1585.
Rose GE, Howard DJ, Watts MR . Periorbital necrotising fasciitis. Eye 1991; 5: 736–740.
Overholt EM, Flint PW, Overholt EL, Murakami CS . Necrotising fasciitis of the eyelids. Otolaryngol Head Neck Surg 1992; 106: 339–344.
Placik OJ, Pensler JM, Kim JJ, Mets MB, Engel JM . Necrotising periorbital cellulitis. Ann Plast Surg 1993; 31: 369–371.
Luksich JA, Holds JB, Hartstein ME . Conservative management of necrotising fasciitis of the eyelids. Ophthalmology 2002; 109: 2118–2122.
Kotb M . 1995. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev 1995; 8: 411–426.
Norrby-Teglund A, Lustig R, Kotb M . Differential induction of Th1 versus Th2 cytokines by group A streptococcal toxic shock syndrome isolates. Infect. Immun 1997; 65: 5209–5215.
Nooh MN, Nookala S, Kansal R, Kotb M . Individual Genetic Variations Directly Effect Polarization of Cytokine Responses to Superantigens Associated with Streptococcal Sepsis: Implications for Customized Patient Care. J Immunol 2011; 186: 3156–3163.
Cole JN, Barnett TC, Nizet V, Walker MJ, Molecular insight into invasive group A streptococcal disease Nat Rev Microbiol 2011; 16: 724–736.
Nowak JE, Wheeler DS, Harmon KK, Wong HR . Admission chemokine (C-C motif) ligand 4 levels predict survival in pediatric septic shock. Pediatr Crit Care Med 2010; 11: 213–216.
Thair SA, Walley KR, Nakada TA, McConechy MK, Boyd JH, Wellman H, Russell JA . A single nucleotide polymorphism in NF-κB inducing kinase is associated with mortality in septic shock. J Immunol 2011; 186: 2321–2328.
Nakada TA, Russell JA, Boyd JH, Walley KR . IL17A genetic variation is associated with altered susceptibility to Gram-positive infection and mortality of severe sepsis. Crit Care 2011; 15: R254.
Cornell TT, Wynn J, Shanley TP, Wheeler DS, Wong HR . Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics 2010; 125: 1248–1258.
Martín-Loeches I, Solé-Violán J, Rodríguez de Castro F, García-Laorden MI, Borderías L, Blanquer J, Rajas O, Briones ML, Aspa J, Herrera-Ramos E, Marcos-Ramos JA, Sologuren I, González-Quevedo N, Ferrer-Agüero JM, Noda J, Rodríguez-Gallego C . Variants at the promoter of the interleukin-6 gene are associated with severity and outcome of pneumococcal community-acquired pneumonia. Intensive Care Med 2012; 38: 256–62.
Allen A, Obaro S, Bojang K, Awomoyi AA, Greenwood BM, Whittle H et al. Variation in Toll-like receptor 4 and susceptibility to group A meningococcal meningitis in Gambian children. Pediatr Infect Dis J. 2003; 22: 1018–1019.
Nooh MM, Nookala S, Kansal R, Kotb M . Individual genetic variations directly effect polarization of cytokine responses to superantigens associated with streptococcal sepsis: implications for customized patient care. J Immunol 2011; 186: 3156–3163.
Nooh MM, El-Gengehi N, Kansal R, David CS, Kotb M . HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J Immunol 2007; 178: 3076–3083.
Ustin JS, Malangoni MA . Necrotising soft-tissue infections. Crit Care Med 2011; 39: 2156–2162.
Aziz RK, Kotb M . Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis 2008; 14: 1511–1517.
Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 2000; 25: 187–191.
Javor J, Bucova M, Cervenova O, Kralinsky K, Sadova E, Suchankova M, Liptakova A . Genetic variations of interleukin-8, CXCR1 and CXCR2 genes and risk of acute pyelonephritis in children. Int J Immunogenet 2012; 39: 338–45.
Mehrotra S, Fakiola M, Oommen J, Jamieson SE, Mishra A, Sudarshan M, Tiwary P, Rani DS, Thangaraj K, Rai M, Sundar S, Blackwell JM . Genetic and functional evaluation of the role of CXCR1 and CXCR2 in susceptibility to visceral leishmaniasis in north-east India. BMC Med Genet 2011; 15: 162.
de Jong R, Altare F, Haagen I, Elferink DG, Boer T, van Breda Vriesman PJ et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 1998; 280: 1435–1438.
Acknowledgements
Mr Geoffrey Rose receives some funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mutamba, A., Verity, D. & Rose, G. ‘Stalled’ periocular necrotising fasciitis: early effective treatment or host genetic determinants?. Eye 27, 432–437 (2013). https://doi.org/10.1038/eye.2012.285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.285