Abstract
Purpose
To assess the quantitative and morphological changes of the macula in response to macular grid laser for diabetic macular oedema (DMO) using optical coherence tomography (OCT).
Patients and Methods
Cirrus OCT macular cube scans of 30 eyes of 25 patients were retrospectively analysed before and 4 months after macular grid laser for diffuse DMO. The oedema was quantified and response evaluated in the nine early-treatment diabetic retinopathy study (ETDRS) zones of the macula. Post-laser OCT changes were compared with the baseline features, including morphology patterns, changes in both logarithmic transformed (logOCT) and standardised average macular thickness (AMT), total macular volume, number of parafoveal quadrants involved, and the presence of intact 3rd hyper-reflective band (HRB).
Results
The rate of change of retinal thickness in response to laser was maximum in the central (8.17%) and perifoveal inferior quadrants (0.04%). Diffuse retinal thickening on OCT responded best to treatment. The AMT of 300–350 μm had the worst response (+0.94%). Eyes with less than four quadrants of oedema showed good response. Disrupted HRB was associated with poor visual gain (−0.33 ETDRS letters).
Conclusion
The topographic location of oedema on the retinal map and the morphological patterns of the oedema on OCT are useful predictors of treatment response in diffuse DMO.
Similar content being viewed by others
Introduction
Diabetic macular oedema (DMO) is a leading cause of blindness among the working population.1 The early-treatment diabetic retinopathy study (ETDRS) group showed that in eyes with clinically significant macular oedema, macular laser reduced the risk of moderate visual acuity (VA) loss (defined as a loss of 15 or more letters) by approximately 50% (from 24 to 12%) after 3 years.2 Clinically significant macular oedema was defined on the basis of stereoscopic fundus photography as retinal thickening within 500 μm of the centre of the macula or hard exudates within 500 μm from the centre of the macula with thickening of the adjacent retina or a zone of retinal thickening, 1 disc area or larger, any portion of which is located within 1 disc diameter from the centre of the macula.2
Fluorescein angiography further differentiates DMO into three types: focal macular oedema when the oedema is localised, diffuse macular oedema when nonspecific leakage is noted at the macula, and ischaemic macular oedema when the oedema is associated with an enlarged and/or disrupted foveal avascular zone. Recent evidence from randomised controlled trials on DMO shows that characteristics of DMO described as focal and diffuse do not correlate with VA or response to treatment.3 So, the Diabetic Retinopathy Clinical Research Network (http://DRCR.net) investigators concluded that new classifications that better characterises the response to treatment are required.3
With the advent of optical coherence tomography (OCT), several investigators have classified DMO on the basis of the retinal map and cross-sectional appearance of the retina on OCT.4, 5 The spectral domain OCT allows better characterisation of the retinal morphology. The morphological patterns of DMO on OCT are generally classified into diffuse retinal thickening (DRT), cystoid macular oedema (CMO), subretinal detachment (SRD), and vitreomacular interface abnormalities.4, 5, 6, 7 Similarly, DMO has been classified on the basis of the area of retinal thickness on the retinal thickness map into diffuse parafoveal thickening defined as having all four parafoveal OCT quadrants greater than 300 μm and focal oedema when fewer than four quadrants are greater than 300 μm.8
In clinical trials, a central macular thickness of 300 μm on Stratus OCT is often taken as the cutoff point to diagnose DMO.9 However, in real life, such numerical values are often not considered. Instead, macular laser is done for any thickening with a clinical diagnosis of clinically significant macular oedema and associated functional loss.
As the response of various grades of thickness to photocoagulation differs, the mean change of central subfield thickness over time in units of logOCT has been shown to provide a better sense of proportionate treatment effects across a wide variety of baseline retinal thicknesses than the actual mean change in micrometres.10 Similarly, absolute values are more reliable indicator in eyes with mild thickening, whereas relative thickness is a more reliable outcome measure in eyes with more severe retinal thickening, and changes in macular volume are a sensitive measure in diffuse macular oedema.11 Standardisation of OCT changes on the basis of normative data has also been suggested as a reliable method.12
In this study, we applied the different outcome measures suggested to examine the effect of modified grid laser photocoagulation on both morphological and quantitative parameters of OCT with a view to understand the predictive factors of macular grid laser that could be translated to real-life settings.
Patients and methods
This study retrospectively analysed the preoperative and 4 months postoperative OCT parameters of 30 eyes of 25 consecutive patients with diffuse DMO that were treated with modified grid laser photocoagulation in the Department of Ophthalmology, King's College Hospital, London. The project was approved by the Chair of the Ethics Committee and registered with the clinical effectiveness department of King's College Hospital. The study protocol adhered to the tenets of the Declaration of Helsinki. Inclusion criteria for the study were patients with the presence of clinically significant macular oedema confirmed as diffuse macular oedema on fluorescein angiography with no previous laser photocoagulation or pharmacologic intervention within 3 months of inclusion.
Exclusion criteria included OCT evidence of vitreomacular interface abnormalities and patients with OCT scan signal of less than 6. Eyes with macular oedema due to other conditions such as venous occlusion were also excluded. All individual scans from each OCT session were manually inspected to ensure proper delineation of the internal limiting membrane and retinal pigment epithelium. Eyes with significant hard exudates that caused improper delineation of the internal limiting membrane/retinal pigment epithelium by the Cirrus HD-OCT software were excluded.
OCT
All scans were performed using the Cirrus OCT version 3.0.0.64 software (Cirrus OCT, Carl Zeiss Meditec, Dublin, CA, USA). Each study eye was pharmacologically dilated before the procedure and OCT scanning was performed using the 512 × 128 scan pattern, where a 6 × 6-mm macular cube was scanned with 128 horizontal B-scan lines, each consisting of 512 A-scans per line (total of 65 536 sampled points). All scans were obtained by experienced photographers who have been certified for OCT scan capture by image-reading centres for clinical studies.
Determination of quantitative parameters
Two quantitative OCT outcomes average macular thickness (AMT) and total macular volume were measured automatically using OCT retinal-mapping software. The AMT is defined as the mean retinal thickness in the circular zone of diameter 1 mm centred on the fovea, and total macular volume as the volume (mm3) of the nine ETDRS subfields covered in the macular scan pattern. The baseline AMT was further classified into three groups: baseline AMT <300 μm, 300–350 μm, and more than 350 μm to note the response rate of different thickness of retina to laser photocoagulation.
We also recorded the average (interpolated) retinal thickness in each of the nine ETDRS subfields as shown in the macular thickness map (Figure 1) to assess the regional changes in retinal thickness following laser therapy.
In order to accurately assess the treatment outcome on retinal thickness, a logarithmic transformation of the OCT central subfield macular thickness (CSMT) measurements (logOCT) was done by taking the log base 10 of the ratio of the CSMT divided by 200 and rounding to the nearest hundredth value. Change in logOCT from baseline was calculated for the follow-up visit by subtracting the change in the baseline logOCT from the follow-up visit logOCT and rounding to the nearest hundredth value.10
Besides, the absolute change in various zones was calculated by subtracting the baseline CSMT from the follow-up thickness. The relative change in thickness is the absolute change divided by the baseline thickness × 100%. The standardized change (SCMT) is the actual change in CSMT (the initial pretreatment thickness minus the post-treatment thickness) using OCT and dividing that value by the potential change (the initial pretreatment thickness minus the normal thickness based on normative data) to provide the total improvement in macular oedema as a percentage.12
Determination of qualitative parameters
Retinal morphology were also assessed using the macular cross-sectional OCT images indicating the reflectivities of retinal structures, and these were classified into three patterns as described by Kim et al.2
The DRT pattern was characterised by a sponge-like retinal swelling of macula with reduced intraretinal reflectivity. The CMO pattern was characterised by intraretinal cystoid spaces of low reflectivity with highly reflective septa separating cystoid-like cavities in the macular area. The SRD pattern was characterised by a shallow elevation of the retina, with an optically clear space between the retina and the retinal pigment epithelium. The OCTs with any vitreomacular interface abnormalities pattern, such as epiretinal membrane, vitreomacular traction, or both were excluded. Epiretinal membranes were deemed present if one or more of the following conditions were met: a macular pseudohole, a difference in optical reflectivity between membrane and retina, a visible membrane tuft or edge.6 The vitreomacular tractions were considered to be present when a highly reflective band was observed on the surface of the retina at specific sites and elevated off the surface elsewhere, whether continuous or not with the posterior vitreous surface.7
Our definition of DRT allowed only pure DRT. If DRT and CMO or SRD were present together, the classifications were CMO or SRD, respectively; and when DRT, CMO, and SRD were present, the classification was SRD. This classification was on the basis of the fact that eyes with SRD carry the worst prognosis. The proportion of persistent DMO patients with different OCT features was evaluated in our study population. Persistent DMO was defined as an increase in baseline thickness after grid laser photocoagulation treatment at 4 months.
Hyper-reflective band (HRB)
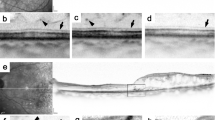
With high resolution and contrast, OCT allows us to evaluate the integrity of the photoreceptors by assessing the status of the third HRB.13, 14, 15, 16 On OCT imaging, the HRB is identified as a distinct band just above the high-reflectance layer of the retinal pigment epithelium–choriocapillaris complex, best detected in greyscale mode (Figure 2).17 The HRB was analysed in the area corresponding to the central 3 mm grid of the ETDRS chart and classified into three groups: intact (group 1), disrupted (group 2), or absent (group 3), depending on the reflectivity and continuity of the band under high contrast settings.
All morphological classification inclusive of the HRB band was graded by two graders (RV and SK) and disagreements were resolved by open discussion with a third investigator (SS), and reclassifications were made only when all parties agreed.
Visual acuity (VA)
The VA was recorded in a busy clinical setting by uncertified staff using Snellen's chart and then converted to ETDRS letters. So, it is likely that the VA is under-estimated and is therefore not used as an outcome measure in this study, except for the correlation with HRB as HRB is regarded as a surrogate marker of visual function.
Retinal photocoagulation
Macular grid photocoagulation was performed following the modified ETDRS laser protocol.18 A grid pattern with barely visible laser burns was applied on the entire oedematous area with a 500-μm safety distance towards the foveal centre. Conventional double frequency Nd YAG 532 nm laser was used with a spot size of 75–125 μm.
Statistical methods
For all quantitative data, t-tests were done between baseline and follow-up measurements and all P-values reported are two sided. The statistical analysis was performed using statistical software (Prism, ver. 4; Graph-Pad (La Jolla, CA, USA) 2002–2005); the criterion for statistical significance was P≤0.05.
Results
Table 1 summarises the baseline features of the subjects.
Change in retinal thickness following macular laser
The CSMT ranged from 270-571 μm pre laser and 270-598 post laser. Similarly, the AMT ranged from 270-398 at baseline and 270–405 post lasers, showing the wide variability in the OCT measurements. Of note, the pattern of relative and absolute change as shown in Figure 3 were identical in both eyes and showed a characteristic pattern of maximum response at the central and inferior perifoveal area. The regions that were least responsive to laser were parafoveal superior, nasal quadrants, and perifoveal temporal quadrants. Other regions showed moderate response (Figure 3).
LogOCT
There was no change in the mean logOCT of CSMT at baseline and at follow-up. However 39.13% eyes showed one-step change in logOCT and 8.7% eyes showed a two-logOCT change.
Qualitative and quantitative response to macular grid laser
The maximum reduction in average retinal thickness following laser was observed in eyes with AMT >350 μm and in eyes with less than four quadrant parafoveal thickening. The response to laser was best in eyes with DFT and cystoid oedema was least responsive (Table 2).
Changes in HRB
In all, 60% of the eyes had an intact HRB with a mean VA of 72.89 ETDRS letters, 20% of the eyes had a disrupted HRB with a mean VA of 67.5 letters, and 20% had an absent HRB with a mean VA of 57.33 letters. At 4 months, the mean gain in VA in the group with intact HRB was 2.89 letters, the group with disrupted HRB lost 0.33 letters, and the group with the absent HRB gained 0.67 letters. Disruption of HRB was not associated with any morphological pattern of oedema.
Persistent macular oedema
An increase in macular thickness was observed in five eyes at 4 months after laser treatment. Three of these eyes had centre-involving oedema, four had baseline DRT, and three of these eyes had less than four quadrant involvement.
Intergrader agreement
Kappa stats. Kappa stats for intergrader agreement on pre- and postlaser classifications on morphology and HRB band showed very good agreement; weighted Kappa was 0.980 and 0.936, respectively.
Changes in HBA1C
Data on HBA1C was available in only 40% of the patients and the mean was 8.87.
Discussion
Our study explored the topographic response of diffuse macular oedema to modified grid laser. We analysed several qualitative and quantitative measures to understand the predictive parameters that would help in routine clinical practice. The relative change in thickness was maximum in the CSMT in centre-involving macular oedema. Change in macular volume is more useful to assess response to non-central macular oedema. Absolute change in retinal thickness is a better predictor of response compared with relative change in oedema <300 μm, whereas relative change is a better outcome measure when the macular thickening is >300 μm. Our findings mirror reports from clinical trials.11
There was a zonal predilection for response with the maximal response observed in CSMT. Browning et al19 noted that in treatment of naive eyes with DMO, the fovea (CSMT) was thicker relative to another zone in 26%; the four inner parafoveal zones were thicker relative to other zones in 13%, whereas the four outer zones were thicker relative to other zones in 6%. We noted that CSMT showed the maximum response. Soliman20 noted a similar pattern in the response rate to laser. They found that the effect of photocoagulation was highest at the foveal centre and the effect decreased with increasing eccentricity. The zone-specific response in our series indicates that CSMT and inferior perifoveal zone are the most responsive and the parafoveal superior and nasal being the least responsive. These features are identical in both eyes, suggesting that the response may be because of the papillomacular bundle-sparing effect of the laser technique or regional differences in blood–retinal barrier characteristics or zonal variations in microcirculation, resulting in regional changes in improved oxygen supply post laser. However, there is no convincing evidence to support these differences.21, 22, 23
Another predictor of better anatomical response to laser treatment is the morphological subtype of the oedema on OCT. The morphological categories of our cohort with diffuse macular oedema showed that 57% were DRT, whereas 37% were cystoid in keeping with the observations of Brar et al.24 On correlating the angiographic and OCT appearance of DMO, they observed that angiographic evidence of diffuse macular oedema is most often associated with DRT on OCT, although microcysts are common, whereas cystoid leakage on fluorescein angiography is always associated with cystic changes on spectral domain OCT. The most favourable outcome was noted with the DRT group, whereas CMO were poor responders. Similar results have been obtained with other forms of therapies for DMO,4, 25, 26, 27 suggesting that the vertical cell–cell alignment of the retinal layers is crucial for positive outcomes to any form of therapeutic intervention. In fact, a recent study showed that the cross-sectional area of retinal tissue between the plexiform layers in CMO on OCT is the best indicator of visual function.28
This observation may also be explained by the fact that DRT may represent the milder form of oedema characterised by intracytoplasmic swelling of Muller cells,29 whereas cystoid spaces result from liquefaction necrosis of Muller cells explaining the poor resolution to treatment.30 The number of SRDs in our case series was very small to assess its predictive value. Although SRD is often considered a poor prognostic indicator in DMO, previous studies on the prognostic value of SRD in DMO have reported conflicting observations.4, 31
Localised macular oedema (less than four parafoveal quadrants) responded better to grid laser than when all four quadrants were involved, supporting a previous study.8 Our study also showed that eyes with moderate AMT of 300–350 μm had the worse response to laser, unlike the findings noted by Estabrook et al.32 We hypothesise that moderate thickening do not benefit from a quick response compared with those with <300 μm oedema and those with severe oedema of >350 μm thickening. A larger sample size will be required to substantiate this finding.
Another prognostic indicator is an intact HRB layer at the fovea. This study showed that eyes with absent or disrupted HRB at baseline had a poorer visual outcome compared with eyes with intact HRB, supporting previous observations that the HRB layer is a valid OCT-derived surrogate marker for visual function in DMO and many other retinal diseases.33, 34, 35, 36, 37, 38, 39 This study reverberates several recent reports that emphasised the correlation of an intact HRB layer with better VA in DMO as well as other retinal diseases.
The strength of this study is that we assessed both quantitative and morphological response to laser treatment using spectral domain OCT. The intergrader agreement is good, suggesting that these parameters could be used in clinical practice with no additional software or manual measurements. The patients included in this series are representative of a real-life ‘see and treat’ retinal clinic.
Our study has a number of limitations related to its retrospective nature. We did not consider the correlation of OCT changes with functional response. The sample size is relatively small, and the overall glycaemic control of these patients was suboptimal compared with those recruited in clinical trials. Patients with HBA1C of 8 or above have increased macular thickness that reduces with strict blood-sugar control.40 There may also be additional unknown confounders such as blood pressure that have not been considered in this study.
In conclusion, several OCT-derived parameters observed in this study have added both qualitative and quantitative dimension in the assessment of DMO and may even reduce the need for fluorescein angiography. Despite the observed correlations, much of the variability in the natural history and therapeutic response of DMO still remains and should continue to be the target of future studies.

References
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL . The Wisconsin epidemiologic study of diabetic retinopathy. IV Diabetic macular oedema. Ophthalmology 1984; 91: 1464–1474.
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular oedema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985; 103: 1796–1806.
Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU. Diabetic Retinopathy Clinical Research Network. Diabetic macular oedema: what is focal and what is diffuse? Am J Ophthalmol 2008; 146 (5): 649–655. e1–6.
Kim NR, Kim YJ, Chin HS, Moon YS . Optical coherence tomographic patterns in diabetic macular oedema: prediction of visual outcome after focal laser photocoagulation. Br J Ophthalmol 2009; 93 (7): 901–905.
Otani T, Kishi S, Maruyama Y . Patterns of diabetic macular oedema with optical coherence tomography. Am J Ophthalmol 1999; 127: 88–93.
Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996; 103: 2142–2151.
Ghazi NG, Ciralsky JB, Shah SM, Campochiaro PA, Haller JA . Optical coherence tomography findings in persistent diabetic macular oedema: the vitreomacular interface. Am J Ophthalmol 2007; 144: 747–754.
Barkmeier AJ, Nicholson BP, Akduman L . Effectiveness of laser photocoagulation in clinically significant macular edema with focal versus diffuse parafoveal thickening on optical coherence tomography. Ophthalmic Surg Lasers Imaging 2009; 40 (5): 472–479.
Lövestam-Adrian M, Holm K . Multifocal electroretinography amplitudes increase after photocoagulation in areas with increased retinal thickness and hard exudates. Acta Ophthalmol 2010; 88 (2): 188–192.
Ferris III FL, Miller KM, Glassman AR, Beck RW. Diabetic Retinopathy Clinical Research Network. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology 2010; 117 (8): 1512–1516.
Browning DJ, Glassman AR, Aiello LP, Bressler NM, Bressler SB, Danis RP et al. Diabetic Retinopathy Clinical Research Network. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology 2008; 115 (8): 1366–1371, 1371.e1.
Chan A, Duker JS . A standardized method for reporting changes in macular thickening using optical coherence tomography. Arch Ophthalmol 2005; 123 (7): 939–943.
Ota M, Tsujikawa A, Murakami T, Kita M, Miyamoto K, Sakamoto A et al. Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol 2007; 91 (12): 1575–1576.
Costa RA, Calucci D, Skaf M, Cardillo JA, Castro JC, Melo Jr LA et al. Optical coherence tomography 3: automatic delineation of the outer neural retinal boundary and its influence on retinal thickness measurements. Invest Ophthalmol Vis Sci 2004; 45: 2399–2406.
Chen TC, Cense B, Miller JW, Rubin PA, Deschler DG, Gragoudas ES et al. Histologic correlation of in vivo optical coherence tomography images of the human retina. Am J Ophthalmol 2006; 141: 1165–1168.
Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL . The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2005; 46: 3349–3354.
Murakami T, Tsujikawa A, Ohta M, Miyamoto K, Kita M, Watanabe D et al. Photoreceptor status after resolved macular oedema in branch retinal vein occlusion treated with tissue plasminogen activator. Am J Ophthalmol 2007; 143: 171–173.
Aiello LP, Edwards AR, Beck RW, Bressler NM, Davis MD, Ferris F et al. Diabetic Retinopathy Clinical Research Network. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2010; 117 (5): 946–953.
Browning DJ, Fraser CM . Regional patterns of sight-threatening diabetic macular edema. Am J Ophthalmol 2005; 140 (1): 117–124.
Soliman W, Sander B, Soliman KA, Yehya S, Rahamn MS, Larsen M . The predictive value of optical coherence tomography after grid laser photocoagulation for diffuse diabetic macular ooedema. Acta Ophthalmol 2008; 86: 284–291.
Tam J, Martin JA, Roorda A . Noninvasive visualization and analysis of parafoveal capillaries in humans. Invest Ophthalmol Vis Sci 2010; 51 (3): 1691–1698.
Kimura I, Shinoda K, Tanino T, Ohtake Y, Mashima Y, Oguchi Y . Scanning laser Doppler flowmeter study of retinal blood flow in macular area of healthy volunteers. Br J Ophthalmol 2003; 87: 1469–1473.
Hudson C, Flanagan JG, Turner GS, Chen HC, Rawji MH, McLeod D . Exaggerated relative nasal-temporal asymmetry of macular capillary blood flow in patients with clinically significant diabetic macular ooedema. Br J Ophthalmol 2005; 89: 142–146.
Brar M, Yuson R, Kozak I, Mojana F, Cheng L, Bartsch DU et al. Correlation between morphologic features on spectral-domain optical coherence tomography and angiographic leakage patterns in macular edema. Retina 2010; 30 (3): 383–389.
Lee CM, Olk RJ . Modified grid laser photocoagulation for diffuse diabetic macular oedema. Long-term visual results. Ophthalmology 1991; 98: 1594–1602.
Brasil OF, Smith SD, Galor A, Lowder CY, Sears JE, Kaiser PK . Predictive factors for short-term visual outcome after intravitreal triamcinolone acetonide injection for diabetic macular oedema: an optical coherence tomography study. Br J Ophthalmol 2007; 91: 761–765.
Guthoff R, Schrader W, Hennemann K, Meigen T, Göbel W . Prognostic factors for visual outcome after intravitreal drug therapy for chronic diabetic macular oedema. Klin Monbl Augenheilkd 2010; E-pub ahead of print 1 July 2010.
Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford M, Marshall J . Optical coherence tomography may be used to predict visual acuity in patients with macular oedema. Invest Ophthalmol Vis Sci 2010; E-pub ahead of print 10 June 2010.
Yanoff M, Fine BS, Brucker AJ, Eagle Jr RC . Pathology of human cystoid macular oedema. Surv Ophthalmol 1984; 28: 505–511.
Fine BS, Brucker AJ . Macular oedema and cystoid macular oedema. Am J Ophthalmol 1981; 92: 466–481.
Ozdemir H, Karacorlu M, Karacorlu SA . Regression of serous macular detachment after intravitreal triamcinolone acetonide in patients with diabetic macular edema. Am J Ophthalmol 2005; 140: 251–255.
Estabrook EJ, Madhusudhana KC, Hannan SR, Newsom RS . Can optical coherence tomography predict the outcome of laser photocoagulation for diabetic macular edema? Ophthalmic Surg Lasers Imaging 2007; 38 (6): 478–483.
Alasil T, Keane PA, Updike JF, Dustin L, Ouyang Y, Walsh AC et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology 2010; 117 (12): 2379–2386.
Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR . The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 2010; 150 (1): 63–67. e1.
Monnet D, Levinson RD, Holland GN, Haddad L, Yu F, Brézin AP . Longitudinal cohort study of patients with birdshot chorioretinopathy: III macular imaging at baseline. Am J Ophthalmol 2007; 144: 818–828.
Oishi A, Otani A, Sasahara M, Kojima H, Nakamura H, Kurimoto M et al. Photoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosa. Eye 2009; 23: 1411–1416.
Ergun E, Hermann B, Wirtitsch M, Unterhuber A, Ko TH, Sattmann H et al. Assessment of central visual function in Stargardt′s disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 2005; 46: 310–316.
Sayanagi K, Sharma S, Kaiser PK . Photoreceptor status after antivascular endothelial growth factor therapy in exudative age-related macular degeneration. Br J Ophthalmol 2009; 93: 622–626.
Hayashi H, Yamashiro K, Tsujikawa A, Ota M, Otani A, Yoshimura N . Association between foveal photoreceptor integrity and visual outcome in neovascular age-related macular degeneration. Am J Ophthalmol 2009; 148: 83–89.
Chou TH, Wu PC, Kuo JZ, Lai CH, Kuo CN . Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye 2009; 23 (6): 1360–1363.
Acknowledgements
We thank Matthew Richardson and Richard Leung, the photographers involved in this study. This work has been presented as a poster at ARVO 2010 Annual Meeting Fort Lauderdale, FL, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S Sivaprasad has received travel grants, research grants and participated in advisory board meetings for Pfizer, Novartis, and Allergan.
Rights and permissions
About this article
Cite this article
Vemala, R., Koshy, S. & Sivaprasad, S. Qualitative and quantitative OCT response of diffuse diabetic macular oedema to macular laser photocoagulation. Eye 25, 901–908 (2011). https://doi.org/10.1038/eye.2011.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.84
Keywords
This article is cited by
-
Bridging the resources gap: deep learning for fluorescein angiography and optical coherence tomography macular thickness map image translation
BMC Ophthalmology (2022)
-
RETRACTED ARTICLE: Cental macular thickness in patients with type 2 diabetes mellitus without clinical retinopathy
BMC Ophthalmology (2013)