Abstract
Purpose
Given the Veterans Affairs Boston Healthcare System's recent introduction of single-use Tonosafe disposable tonometer prisms as an alternative to Goldmann applanation tonometers (GATs), this study had two aims: to conduct a large-scale quality assurance trial to assess the reliability of intraocular pressure (IOP) measurements of the Tonosafe disposable tonometer compared with GAT, particularly at extremes of pressure; to evaluate the suitability of Tonosafe disposable tonometer prisms as an acceptable substitute for GATs and for clinic-wide implementation in an academic tertiary referral setting.
Methods
Ophthalmology resident physicians measured the IOPs of patients in general and specialty eye clinics with the Tonosafe disposable tonometer and GAT. Tonosafe test–retest reliability data were also collected. A retrospective review of patient charts and data analysis were performed to determine the reliability of measurements.
Results
The IOPs of 652 eyes (326 patients) were measured with both GAT and Tonosafe, with a range of 3–34 mm Hg. Linear regression analysis showed R=0.93, slope=0.91, both of which supported the proposed hypothesis, and the y-intercept=−1.05 was significantly different from the hypothesized value. The Tonosafe test–retest repeatability (40 eyes of 40 patients), r=0.977, was very high, which was further supported by linear regression slope=0.993, y-intercept=0.118, and a Tonosafe repeatability coefficient of 2.06, similar to GAT repeatability.
Conclusions
The IOP measurements by Tonosafe disposable prisms correlated closely with Goldmann measurements, with similar repeated measurement variability to GAT. This suggests that the Tonosafe is an acceptable substitute for GAT to measure IOP in ophthalmology clinic settings.
Similar content being viewed by others
Introduction
Outbreaks of epidemic ocular infections have occurred in ophthalmology clinics in medical centres around the world in the past several years,1, 2, 3 with insufficient disinfection of Goldmann tonometer prisms being implicated as a potential source of transmission.4 Epidemiological and clinical studies of epidemic keratoconjunctivitis outbreaks in ophthalmology clinics have demonstrated that tonometer tips, contaminated eye drops, and contact with infected healthcare workers are vehicles for transmission of adenovirus serotypes.3, 5, 6 Adenovirus can survive for 7–14 days at room temperature on a dry surface and is resistant to 70% isopropyl alcohol,7 which is often used in busy clinic settings.8 Improper sterilization techniques also increase the risk of corneal injury and burn secondary to residual cleansing solution on reusable Goldmann prisms.9
Therefore, in 2009, the Veterans Affairs Boston Healthcare System (VABHS) introduced single-use Tonosafe disposable tonometer prisms (Clement Clarke, Columbus, OH, USA) as an alternative to Goldmann tonometer prisms for measuring intraocular pressure (IOP) across all general and specialty eye clinics. The Tonosafe tonometer head, which consists of a precision-molded plastic holder and an acrylic disposable optical doubling applanating prism,10 has similar properties to the Goldmann tonometer. The Tonosafe holder and prism combined mass is equivalent to that of the Goldmann, 1.65±0.05 g, and has an identical applanating surface area.11 The Goldmann applanation tonometry method has inherent errors in IOP measurement accuracy, due to the variability in corneal thickness and rigidity, as well as the tear film surface tension. These are impacted by adhesion properties and the prism material, which exert a pressure that offsets the effect of corneal resistance.12 The acrylic Tonosafe tip may affect the adhesion properties of the tear film, leading to measurement errors as well.
Previous smaller studies have investigated the accuracy of the Tonosafe disposable tonometer prism compared with the Goldmann tonometer prism, with majority of IOP measurements being in the normal range,13 thereby lacking generalizability at higher pressures. This large-scale quality assurance study was conducted to assess the reliability of IOP measurements of the Tonosafe disposable compared with Goldmann tonometer prisms, particularly at the extremes of intraocular pressure, and to assess the suitability of the Tonosafe disposable tonometer prisms for clinic-wide implementation in an academic tertiary referral setting, as an acceptable substitute for GAT to measure IOP in ophthalmology clinics.
Materials and methods
Research design
This is an IRB-approved retrospective chart review of patients in the VA Boston Healthcare System (VABHS). We certify that all applicable institutional and governmental regulations concerning the ethical use of patient data were followed for this research. All patients were examined in the VABHS general and specialty eye clinics. Patients with corneal abnormalities including ulcers, scars, or keratoprosthesis were excluded from the study. Female patients were also excluded because the majority of ophthalmology patients in VABHS were male.
Methodology
The IOPs of patients were measured using both the Goldmann applanation tonometer prisms and the newly introduced, single-use Tonosafe disposable tonometer prisms. The residents were instructed to check IOP using both devices for each patient, while waiting for at least 1 min between measurements to avoid erroneously lower pressure readings from repeated tonometry.14 When aligning the mires, the residents were instructed to look only through the slit lamp, only looking at the dial ultimately to round to the nearest integer.
Residents alternated the testing method used first on each patient for counterbalancing. The residents recorded both measurements and the order in which the testing was performed in the patient's electronic medical record. Additional information—if applicable—such as patient positioning, variability, and eyelid squeezing were also documented in the chart.
After use, the Tonosafe heads were disposed and the Goldmann tonometer heads were disinfected by the Sterilization and Processing Department (SPD) according to the VABHS protocol: immersion of the Goldmann tip in 10% bleach for 10 min, followed by thorough rinsing with tap water for 60 s, then rinsing with sterile water, and finally wiping it dry.15 The cleaned Goldmann tips were returned to the ophthalmology department for reuse in the clinic.
For an additional group of patients, residents were instructed to measure the IOP twice for the right eye using Tonosafe, waiting for approximately 1 min between measurements with the same instructions, as indicated above, to minimize the bias.
The electronic medical records of patients seen over the study period were retrospectively reviewed, with IOP measurement data and resident level of experience noted.
Statistical analysis
The IOP measurements by Tonosafe disposable and Goldmann applanation device were compared using Pearson’s product moment correlation and linear regression analysis. Tonosafe test–retest reliability, or repeatability, was also assessed to evaluate the variability with repeated measurements for comparison with GAT. Subgroup analyses by extremes of pressure and by the experience of residents were performed.
Results
The intraocular pressures of 708 eyes of 382 patients were measured by both Tonosafe and Goldman applanation, with an IOP range of 0–72 mm Hg. The data were cleaned by eliminating any patient with one or more missing data points and any outliers that fell beyond the 95% confidence interval (N=2). The measurements were then pooled across the eye and sequenced, with a new sample size of 652 eyes (326 patients), with an IOP range of 3–33 mm Hg for GAT and 3–34 mm Hg for Tonosafe.
To assess the equivalence of the Tonosafe and Goldmann, linear regression analysis was performed (Figure 1). The hypothesis that there would be equivalence between these two sets of measurements was assessed by three criteria: (a) strength of relationship (Pearson's product moment correlation), R≥0.95, (b) slope=1.0, and (c) intercept=0.0. The regression analysis showed R=0.93, a linear regression slope=0.91, both of which supported the hypothesis, and y-intercept=−1.05, which was statistically different from the hypothesis. However, further analyses were conducted to determine the clinical equivalence and importance of this difference.
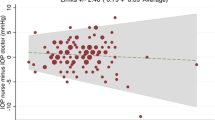
To assess for clinical agreement between the two devices, Bland–Altman plot from the pooled data was constructed (Figure 2). The difference between IOP measurements by Tonosafe and Goldmann (Y-axis) was plotted against the mean of the IOP measured with the two methods (X-axis). This analysis showed R=0.037 (P=0.35) and slope=0.05 (P=0.983), both of which are consistent with the regression results.
Correlation between Tonosafe and Goldmann measurements increased as level of experience increased: first-year (r=0.91), second-year (r=0.94) and third-year resident physicians (r=0.96).
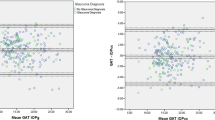
Tonosafe right eye measurements were repeated twice in 40 patients to assess the repeatability of this device, for comparison with GAT. This showed a very high test–retest reliability, r=0.977, further supported by linear regression slope=0.993, y-intercept=0.118 (Figure 3). As seen in the Bland–Altman plot for Tonosafe repeatability (Figure 4), there was a mean difference=0.01, SD=1.03 with R=0.031, slope=−0.007, all of which support high test–retest reliability. For comparison purposes with Goldmann, the Tonosafe coefficient of repeatability (CR), the 95% confidence limit of the difference in test and retest scores,16 was found to be 2.06, calculated by the following equation:

According to the literature, the GAT CR is 1.6–2.9,17, 18, 19 which is very close. Table 1 compares various parameters for Tonosafe test–retest reliability and GAT test–retest reliability,20, 21, 22 showing similar variability between the two devices.
Discussion
VA Boston Healthcare System practice
Stricter protocols are being established in the VA hospital system surrounding sterilization of reusable medical equipment. The time and logistics required for the recommended multi-step disinfection23 regimen of the Goldmann applanation tonometer poses significant challenges in a busy ophthalmology clinic setting. Several studies have demonstrated the benefits of using disposable tonometer devices to reduce the risk of cross-infection between patients in eye clinics. The transition period before switching entirely from Goldmann tonometers to Tonosafe disposable prisms across all VABHS eye clinics presented a critical window to conduct this study. To our knowledge, this was the largest study to assess the reliability of Tonosafe disposable prisms compared with the Goldmann applanation prism, particularly at extremes of pressure. The introduction of disposable tonometer tips has become an alternative practice in many VA hospitals.
Summary of data
Linear regression analysis was used to determine whether the Tonosafe generates an IOP measurement equivalent to the Goldmann, such that the devices would be essentially interchangeable. In our study, IOP measurements with the Tonosafe disposable correlated closely with the Goldmann tonometer, with a similar slope. Although the y-intercept may suggest that the predicted Tonosafe IOP measurement may be adjusted downwards by approximately 1 mm Hg, this falls within the range of variability inherent in repeated Tonosafe and the GAT measurements. The very high test–retest reliability for the Tonosafe is indeed similar to the GAT's repeatability, as demonstrated in Table 1, and further supported by the Bland–Altman plots. These collective findings from our analyses suggest that Tonosafe may be an acceptable substitute for GAT in ophthalmology clinics.
Our subgroup analyses indicated that correlation between Goldmann and Tonosafe increased as level of provider experience increased.
Cost–benefit analysis of disposable devices
Table 2 includes a comprehensive cost analysis comparing the Goldmann with alternative disposable devices. One UK study found that often only one Goldmann prism was provided per practitioner, thereby reducing the likelihood of adequate soak time between patients.8 In a busy clinic setting, the supply must be greatly increased to avoid delay in patient care, with 200 Goldmann prisms required for routine use by practitioners. The VABHS clinic has seen a rate of damage and loss to the tips at 50% with SPD cleaning, creating a need for an additional 100 replacement tips. Cost is not the sole factor determining the use of one device over another; therefore, the information in this table should be utilized in the context of the overall advantages and disadvantages of each, which are shown in Table 3. The cost–benefit analysis shows that the Tonosafe disposable prism may be both a reliable and cost-effective alternative to the Goldmann.
Limitations
One limitation of our study was that data were obtained from retrospective chart review, rather than a prospective trial. Although resident physicians were instructed to wait a minimum of 1 min between methods, this interval was not timed or recorded to avoid disruption of work flow in this setting. The potential for bias also arises as the residents were not masked between intraocular pressure readings; however, the instructions to align mires through the slit lamp only were intended to minimize this bias. Counterbalancing the sequence may have corrected for any significant influence the first reading may have had on the second reading. Also, given the nature of the VABHS patient population, there were too few women to obtain valid results for this group.
Conclusions
The Medical Devices Agency advisory stated that ophthalmic devices that contact the ocular surface should be ‘restricted to single patient use wherever practicable and where this does not compromise clinical outcome.’27 The IOP measurements by Tonosafe disposable prisms correlated closely with Goldmann measurements by linear regression analysis, with similar variability with repeated measurements when compared with GAT. This suggests that the Tonosafe may be an acceptable substitute for GAT to measure IOP in ophthalmology clinic settings. This study also demonstrates the results that a facility can expect to obtain when the Tonosafe is introduced rapidly in an environment with providers with varying levels of experience.

References
Warren D, Nelson K, Farrar J, Hurwitz E, Hierholzer J, Ford E et al. A large outbreak of epidemic keratoconjunctivitis: problems in controlling nosocomial spread. J Infect Dis 1989; 160: 938–943.
Centers for Disease Control, Prevention. Epidemic keratoconjunctivitis in an ophthalmology clinic of California. MMWR 1990; 39: 598–601.
Viney KA, Kehoe PJ, Doyle B, Sheppeard V, Roberts-Witteveen AR, Semirli H et al. An outbreak of epidemic keratoconjunctivitis in a regional ophthalmology clinic in New South Wales. Epidemiol Infect 2008; 136 (9): 1197–1206.
Segal WA . Disinfection of Goldmann tonometers after contamination with Hepatitis C virus. Am J Ophthalmol 2001; 131: 184–187.
Cheung D, Bremner J, Chan JK . Epidemic keratoconjunctivitis—do outbreaks have to be epidemic? Eye 2003; 17: 356–363.
Cillino S, Cassucio A, Giammanco GM et al. Tonometers and infectious risk: myth or reality? Efficacy of different disinfection regimens on tonometer tips. Eye 2007; 21: 541–546.
Buehler JW, Finton RJ, Goodman RA . Epidemic keratoconjunctivitis: report of an outbreak in an ophthalmology practice and recommendations for prevention. Infect Control 1984; 5: 390–394.
Hillier RJ, Kumar N . Tonometer disinfection practice in the United Kingdom: a national survey. Eye 2008; 22: 1029–1033.
Levenson J . Corneal damage from improperly cleaned tonometer tips. Arch Ophthal 1989; 107: 1117.
Goel S, Chua C, Dong B, Butcher M, Ahfat F, Hindi SK et al. Comparison between standard Goldmann applanation prism and disposable applanation prism in tonometry. Eye 2004; 18: 175–178.
Kim P, Lertsumitkul S, Clark M, Gardner L, Macken P . Accuracy of the Tonosafe disposable tonometer head compared to the Goldmann tonometer alone. Clin Exp Ophthalmol 2004; 32: 364–367.
Liu J, Roberts CJ . Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005; 31: 146–155.
Maino AP, Uddin HJ, Tullo AB . A comparison of clinical performance between disposable and Goldmann tonometers. Eye 2006; 20: 574–578.
Whitacre MM, Stein R . Sources of error with use of Golmann-type tonometers. Surv Ophthalmol 1993; 38: 1–30.
Department of Veterans Affairs VA Boston Healthcare System, Standard Operating Procedure (SOP). Re-usable Haag-Streit tonometer prisms, July 2009.
Bland JM, Altman DG . Statistical method for assessing agreement between two methods of clinical measurement. The Lancet 1986; 1: 307–310.
Kotecha A, White ET, Shewry JM, Garway-Heath DF . The relative effects of corneal thickness and age on Goldmann applanation tonometry and dynamic contour tonometry. Br J Ophthalmol 2005; 89: 1572–1575.
Kotecha A, White E, Schlottmann PG, Garway-Heath D . Intraocular pressure measurement precision with the Goldmann applanation, dynamic contour, and ocular response analyzer tonometers. Ophthalmology 2010; 117 (4): 730–737.
Dielemans I, Vingerling JR, Hofman A, Grobbee DE, de Jong PT . Reliability of intraocular pressure measurement with the Goldmann applanation tonometer in epidemiological studies. Graefes Arch Clin Exp Ophthalmol 1994; 232: 141–144.
Sudesh S, Moseley MJ, Thompson JR . Accuracy of Goldmann tonometry in clinical practice. Acta Ophthalmol 1993; 71: 185–188.
Recep OF, Hasiripi H, Vayỳsoglu E, Kalayci D, Sarikatipoglu H . Accurate time interval in repeated Tonometry. Acta Ophthalmol Scand 1998; 76 (5): 603–605.
Moses RA, Liu CH . Repeated applanation tonometry. Am J Ophthalmol 1968; 66: 89–91.
American Academy of Ophthalmology. American Academy of Ophthalmology information statement: minimising transmission of bloodborne pathogens and surface infectious agents in ophthalmic offices and operating rooms. AAO: San Francisco, 2002.
Bhatnagar A, Gupta AK . Disposable devices for measuring intraocular pressure: a clinical study to assess their accuracy. Eye 2005; 19: 752–754.
Kniestedt C, Punjabi O, Lin S, Stamper RL . Tonometry through the ages. Surv Ophthalmol 2008; 53 (6): 568–591.
Maino AP, Morgan LH, Hercules BL, Tullo AB . Are disposable prisms an adequate alternative to standard Goldmann tonometry prisms in glaucoma patients? Ophthalmology 2006; 113(10): 1837–1841.
Medical Devices Agency. MDA AN 1999(04) Single patient use of ophthalmic medical devices: implications for clinical practice. Available at http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/Advicenotices/CON008875.
Acknowledgements
Many thanks to the residents and staff of the Ophthalmology Section, the members of SPD Department, and Gary Park from Research at the VA Boston Healthcare System for their dedication to patient care and safety. Thanks also to P Legutko, PhD, for his additional assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Previous Presentation: This work was presented as a poster at the XIX Biennial Meeting of the International Society of Eye Research, July 18–23, 2010 in Montreal, Canada.
Rights and permissions
About this article
Cite this article
Thomas, V., Daly, M., Cakiner-Egilmez, T. et al. Reliability of tonosafe disposable tonometer prisms: clinical implications from the Veterans Affairs Boston Healthcare System Quality Assurance Study. Eye 25, 651–656 (2011). https://doi.org/10.1038/eye.2011.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.40