Abstract
Purpose
The purpose of this study is to investigate the morphological features of epiretinal membrane (ERM) in the presence or absence of a posterior vitreous detachment (PVD).
Design
Retrospective observational comparative case series.
Methods
This study involved 34 patients in whom the vitreoretinal relationship was confirmed during vitrectomy for treatment of ERM. We analyzed demography, direction of macular folds in fundus photographs, and foveal contours assessed by optical coherence tomography (OCT) in two groups of patients, the posterior vitreous attachment (PVA) group and the PVD group.
Results
Mean age of the 14 patients in the PVA group was 58.2±8.2 years, and that of 20 patients in the PVD group was 66.7±6.7 years (P=0.0023). Funduscopic examination revealed radiating folds in 57.1% of patients in the PVA group and 10% of patients in the PVD group, and a flat-shaped foveal contour was observed on OCT in 71.5% of patients in the PVA group and 25.0% of patients in the PVD group. Significant differences were observed between the PVA group and the PVD group in both direction of the macular folds (P<0.01) and foveal contours (P=0.028).
Conclusion
Patients with ERM and PVA were usually younger than 60 years. Radiating macular folds and flat foveal contour in patients with ERM are highly sensitive and specific findings indicative of PVA.
Similar content being viewed by others
Introduction
Surgical treatment for epiretinal membrane (ERM) includes pars plana vitrectomy and peeling of ERM, with or without removal of internal limiting membrane (ILM). To achieve the surgical endpoint, induction of a posterior vitreous detachment (PVD) is occasionally required before the membrane removal. Preoperative incidence of a PVD has generally been reported in 57 to 90% of patients with idiopathic ERM.1, 2 In our recent studies, PVD was noted in 78.5% of eyes with idiopathic ERM.3 Thus, surgical induction of a PVD during vitreous surgery is not necessary in the majority of ERM cases. However, preexistence of a PVD may affect the method of surgical approach and occurrence of vitrectomy-related complications.3, 4 Induction of a PVD during vitrectomy will result in a significantly higher occurrence of intraoperative or postoperative retinal breaks.5 On the contrary, the preexistence of a posterior vitreous separation might be associated with the presence of peripheral retinal break before operation. Although PVD seems to be closely related to the development of ERM in many of the cases, the existence of ERM even in the absence of PVD implies that the syndrome of ERM may evolve out of diverse pathophysiologic events. In other words, the pathophysiologic and prognostic implications of ERM may differ according to the presence or absence of PVD. Thus, identifying the vitreoretinal relationship in ERM cases seems significant in clinical and pathophysiological perspectives. The diagnosis of PVD in ERM cases principally depends on the identification of Weiss ring on funduscopic examination. The development of optical coherence tomography (OCT) allows a detailed visualization of vitreoretinal relationship in major maculopathies. However, the accurate diagnosis of PVD using OCT in ERM cases is frequently difficult, because OCT cannot differentiate complete PVD from no PVD. Although a preoperative diagnostic evaluation of the vitreoretinal relationship by means of B-scan ultrasonography would be of help, it could be inaccurate.
This study was conducted to elucidate the clinical characteristics of ERM according to the presence or absence of a PVD.
Materials and methods
This was a retrospective, clinical case series of 34 eyes that underwent vitrectomies for treatment of idiopathic ERM between 1 July 2007 and 30 June 2008. During the period of this study, vitrectomies were conducted in a total of 106 eyes with idiopathic ERM. To be included in this study, the subjects should have preoperative OCT (Stratus OCT version A 3.0; Carl Zeiss Meditec, Dublin, CA, USA) and preoperative fundus photography. Documentation showing intraoperative confirmation of whether or not a PVD had been induced was also required. If a patient has undergone surgery for ERM in both eyes, only the first eye to have undergone the surgery was included in this study. Cases involving preoperative retinal breaks, uveitis, trauma, retinal vascular disorders, diabetic retinopathy, history of intraocular surgery (except for cataract surgery), intraocular tumors, and refractive errors of greater than −6.0 diopters, were not included in the study. However, eyes were excluded mainly because of incomplete preoperative data, and/or absent or ambiguous operative documentation regarding PVD. All subjects underwent a complete preoperative evaluation, including best-corrected Snellen visual acuity (BCVA), anterior segment examination, and dilated fundus examination with a 90-diopter lens. Indirect ophthalmoscopic examination with a scleral indentation for identification of possible peripheral retinal problems was conducted preoperatively in all eyes. BCVA values were converted to the logMAR scale for statistical analyses. Institutional review board approval (IRB file number; 2009-08-097) was obtained before patient records were reviewed.
A three-port conventional pars plana vitrectomy, using a 20-gauge (four eyes, 11.8%) or 23-gauge (30 eyes, 88.2%) instrument, was conducted by one surgeon (SWK). Operative procedures included core vitrectomy, induction of a PVD (as needed), and removal of cortical vitreous, as well as peeling of both ERM and ILM. Induction of a PVD was conducted by engaging the attached posterior cortical vitreous with a vent 20 or 23-gauge needle in the area adjacent to the optic disc, and elevating it towards the postero-anterior direction approximately three-disc diameters away from the margin of the optic disc.
The 34 eyes of 34 subjects included in this study were divided into two groups on the basis of the necessity of induction of a PVD during vitreous surgeries. The posterior vitreous attachment (PVA) group included eyes that required induction of a PVD at the time of surgery, and the PVD group included eyes in which the preoperative presence of a PVD had been confirmed during surgery. Intraoperative determination of the presence or absence of a PVD was the only criterion for division of eyes into two groups (PVA group or PVD group).
Preoperative BCVA, duration of visual symptoms, age at the time of surgery, lens status, central macular thickness, postoperative visual outcome, and any adverse events, were analyzed for each group.
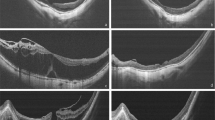
We attempted to find the correlation between vitreoretinal relationship and macular morphology on fundoscopy and OCT. Morphology of macular folds on preoperative fundus photographs was classified into three types: radiating fold, non-radiating fold, and without fold. Retinal folds spreading from the foveal center to the periphery like a ray of sunshine were defined as ‘radiating folds’. Even though the folds were discontiguous, it was considered as ‘radiating’ when these folds were shown in range of more than six clock hours (Figure 1a). A ‘non-radiating folds’ was defined as a macular fold that did not fulfill the criterion of a ‘radiating folds’, including multidirectional or linear folds (Figure 1b). ‘Without fold’ was defined as no conspicuous fold on fundus photograph (Figure 1c). Appearance of ERM on OCT was also classified into three types: flat, convex, and concave thickening of the foveal center (Figure 2). Classification of preoperative macular folds and OCT types was conducted by independent observers, masked to the other clinical findings of each case. Statistical analyses were conducted using SPSS v.13.0 software (SPSS Inc., Chicago, IL, USA). Results were considered significant at P-values < 0.05.
The classification of ERM by funduscopic features. The folds that spread from the foveal center to the periphery like a ray of sunshine are defined as ‘radiating folds’ when they extend at least more than six clock hours range despite their discontinuity (a). Macular folds are defined as ‘non-radiating folds’ that do not fulfill the criterion of ‘radiating folds’ (b). It is defined as ‘without folds’ if no conspicuous folds exist (c).
Results
A total of 34 eyes of 34 patients (18 men, 16 women) were included in this study; there were 17 right eyes and 17 left eyes. Age at the time of surgery ranged from 46 to 82 years (average, 63.2 years). Surgical induction of a PVD was conducted in 14 eyes (PVA group), and pre-existing PVD was confirmed during surgery in 20 eyes (PVD group). Mean age (±SD) of 14 patients in the PVA group was 58.2±8.2 years, and that of 20 patients in the PVD group was 66.7±6.7 years. Patients in the PVA group were statistically younger than patients in the PVD group (P=0.0023, t-test; Table 1). All patients were symptomatic with metamorphopsia and/or blurred vision. Duration of visual symptoms before surgery was 9.6±9.7 months in the PVA group, and 22.1±19.6 months in the PVD group (P=0.0257, Wilcoxon two-sample test). To eliminate differences in age and lens status between the two groups, we analyzed the duration of visual symptoms between the two groups using multiple variable analysis. After adjusting age and lens status, we detected no significant differences in duration of visual symptoms between the two groups (P=0.1060, multiple logistic regression analysis).
Preoperative BCVA (logMAR) was 0.43±0.35 in the PVA group, and 0.39±0.24 in the PVD group (P=1.0000, t-test with Bonferroni's correction). After surgery, BCVA (logMAR) was improved to 0.21±0.18 (P=0.0191, paired t-test) in the PVA group, and 0.21±0.14 (P=0.0029, paired t-test) in the PVD group. However, no significant difference of final mean postoperative BCVA was observed between the two groups (P=1.0000, t-test with Bonferroni's correction).
Preoperatively, 15 eyes (44.1%, 12 of 14 PVA group eyes and 3 of 20 PVD group eyes) were phakic without cataracts, 9 eyes (26.5%, 1 of 14 PVA group eyes and 8 of 20 PVD group eyes) were phakic with lens opacity, and 10 eyes (29.4%, 1 of 14 PVA group eyes and 9 of 20 PVD group eyes) were pseudophakic (P=0.019, Fisher’s exact test). PVD was more common in eyes from which the crystalline lens has been removed (odds ratio=0.094, 95% CI=(0.010, 0.863), P=0.0366, logistic regression analysis). Phacoemulsification and implantation of the posterior chamber intraocular lens combined with vitrectomy were conducted in nine eyes, with preoperatively detected lens opacification (1 of 14 PVA group eyes and 8 of 20 PVD group eyes).
A Weiss ring was observed in 11 eyes (55.0%) in the PVD group during preoperative fundus examination using slit-lamp biomicroscopy. The remaining eyes were ambiguous with regard to existence of a Weiss ring. Radiating folds were observed in eight eyes (57.1%) in the PVA group and two eyes (10.0%) in the PVD group. Non-radiating folds were observed in four eyes (28.6%) in the PVA group and 14 eyes (70.0%) in the PVD group. Of the remaining eyes, two eyes (14.3%) in the PVA group and four eyes (20.0%) in the PVD group did not demonstrate macular folds. Thus, the eyes in PVA group predominantly exhibited radiating macular folds, and distribution of the classification of macular fold on the preoperative fundus photograph was significantly different between the two groups (Fisher's exact test, P<.01). Preoperative OCT examination showed that the shape of the foveal contour was flat in 10 eyes (71.5%) in the PVA group and 5 eyes (25.0%) in the PVD group; convex in 3 eyes (21.4%) in the PVA group and 10 eyes (50.0%) in the PVD group; and concave in one eye (7.1%) of the PVA group and five eyes (25.0%) of the PVD group. These distributions in the shape of the foveal contour observed on OCT were significantly different between the two groups (Fisher's exact test, P=0.028). If both radiating folds and flat foveal contour were noted in one eye, the probability of PVA in this eye would be 83.3%, and in the same combination, the specificity of PVA would be 67.9%. Mean central retinal thickness measured preoperatively was 395.6±103.1 in the PVA group and 401.1±96.8 in the PVD group (Wilcoxon two-sample test, P=0.986).
Discussion
As a result of this study, the presence of radiating retinal folds on funduscopy and flat thickening of the foveal contour on OCT indicate the absence of a PVD with high sensitivity and reasonable specificity. In addition, this study also found that ERM cases not accompanied by a PVD had a particular association with young age <60 years and phakia.
Presence or absence of a PVD must be determined before or during surgery. If no PVD is present, it must be intentionally induced. In such cases, the chance of developing peripheral retinal breaks during surgery becomes significantly high.3, 4 Therefore, special attention should be paid during vitrectomy. On the other hand, eyes with a pre-existing PVD may be accompanied by pre-existing peripheral retinal breaks. Assessment of vitreous architecture is not easy, and accurate clinical diagnosis of a PVD is also not easy. Visualization of a Weiss ring has been traditionally regarded as the hallmark of a PVD. However, it seems to be a rather insensitive marker for identification of a PVD, because preoperative identification of a Weiss ring was noted in only 55% (11 of 20 eyes) of eyes in the PVD group in the current study.
In the eyes of the PVA group, the membrane probably begins to form between the posterior hyaloid and ILM, whereas the vitreous still adheres to the retina. Cells forming the membrane might accumulate in the outermost layer of the posterior hyaloid itself, not over the ILM of the retina. It may be possible that the membrane itself is the thickened posterior hyaloid. In some of the cases of PVA group, we found that the ERM was peeled off during induction of a PVD. In addition, histopathologic features of ERM without a PVD have been reported.5 Transmission electron microscopy revealed that the membranes were collagen of <16 nm in diameter, suggesting that they were consistent with native vitreous collagen.5 If the outermost cortical layer of the vitreous constitutes the ERM, or if adhesion between the vitreous and the ERM is stronger than that between the ERM and ILM, spontaneous peeling of ERMs may occur concurrently with a PVD.6, 7, 8 We reviewed fundus photographs in two published articles that reported spontaneous peeling of ERM.6, 8 Interestingly, radiating macular folds in the fundus photographs before development of a PVD were noted in four of five cases in those articles. However, the mechanism for development of ERM in patients without a PVD remains unclear. In the eyes of the PVA group, these folds were not just a visual interface phenomenon or full thickness retinal folds, but undulations of inner retinal layers on OCT. We could observe that the folds have disappeared in a funduscopic examination after the surgery including ILM peeling.
The tissue from idiopathic macular pucker in children and young adults, in whom the existence of PVA could be presumed, consisted of myofibroblasts, myofibroblastic differentiation of retinal pigment epithelial cells, fibrous astrocytes, and collagen.9 It is interesting to note that myofibroblasts, astrocytes, and fibrocytes, predominate also in vitreomacular traction syndrome,10, 11 whereas retinal pigment epithelial cells are commonly found in ERM.12, 13
Radiating retinal folds in eyes with PVA may represent vitreous traction concentrated in the center of the fold (Figure 3). Thus, ERM in those eyes may not be categorized as a simple idiopathic ERM, but rather as a kind of vitreomacular traction syndrome, due to its adhesion between the vitreous and ERM itself. The hallmark of vitreomacular traction syndrome is the persistent attachment of the vitreous to the macula with an incomplete PVD.14 This traction is thought to be mediated by the vitreous cortex and by fibrocellular proliferation at the vitreoretinal interface. Eyes with vitreomacular traction are associated with more serious morphologic and functional changes than those without.14 Therefore, although further study is required, early surgical intervention should be contemplated in this subset of ERM cases, simulating vitreomacular traction syndrome.
Ripandelli et al15 found that a PVD occurred between 2 days and 26 months after phacoemulsification in 107 of 141 (75.88%) eyes without preoperative PVD. Our results also revealed that a PVD was more frequently observed in pseudophakic eyes (P=0.019). However, in our cases, existence of a PVD before cataract surgery could not be determined.
This study suffers from being retrospective in nature, and from having a small sample size. And the composition of cases in this study may not represent ERMs in general. There are no particular intra- and post-operative risks for eyes in which a PVD did not occur at the time of surgery with respect to the other group. It was caused that the number of patients is small. A larger number of cases would be required. However, if a vitreoretinal surgeon can recognize the PVA in the eyes with ERM, the implementation of vitreous surgery will be possible in more predictable way. In addition, our results raise the possibility that the ERM in the absence of a PVD could be a specific manifestation of vitreomacular traction syndrome.
In conclusion, the main clinical appearance of idiopathic ERM without a PVD includes radiating retinal folds found during fundoscopic examination, and flat thickening of foveal contour on OCT images in younger phakic patients. Such eyes responded well to surgery. Further investigations are warranted for determination of pathogenesis and course in both types of ERMs, with and without a PVD.

References
Hirokawa H, Jalkh AE, Takahashi M, Trempe CL, Schepens CL . Role of the vitreous in idiopathic preretinal macular fibrosis. Am J Ophthalmol 1986; 101: 166–169.
Sidd RJ, Fine SL, Owens SL, Patz A . Idiopathic preretinal gliosis. Am J Ophthalmol 1982; 94: 44–48.
Chung SE, Kim KH, Kang SW . Retinal breaks associated with the induction of posterior vitreous detachment. Am J Ophthalmol 2009; 147: 1012–1016.
Guillaubey A, Malvitte L, Lafontaine PO, Hubert I, Bron A, Berrod JP et al. Incidence of retinal detachment after macular surgery: a retrospective study of 634 cases. Br J Ophthalmol 2007; 91: 1327–1330.
Heilskov TW, Massicotte SJ, Folk JC . Epiretinal macular membranes in eyes with attached posterior cortical vitreous. Retina 1996; 16: 279–284.
Mulligan TG, Daily MJ . Spontaneous peeling of an idiopathic epiretinal membrane in a young patient. Arch Ophthalmol 1992; 110: 1367–1368.
Greven CM, Slusher MM, Weaver RG . Epiretinal membrane release and posterior vitreous detachment. Ophthalmology 1988; 95: 902–905.
Meyer CH, Rodrigues EB, Mennel S, Schmidt JC, Kroll P . Spontaneous separation of epiretinal membrane in young subjects: personal observations and review of the literature. Graefes Arch Clin Exp Ophthalmol 2004; 242: 977–985.
Smiddy WE, Michels RG, Gilbert HD, Green WR . Clinicopathologic study of idiopathic macular pucker in children and young adults. Retina 1992; 12: 232–236.
Gandorfer A, Rohleder M, Kampik A . Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol 2002; 86: 902–909.
Smiddy WE, Green WR, Michels RG, de la Cruz Z . Ultrastructural studies of vitreomacular traction syndrome. Am J Ophthalmol 1989; 107: 177–185.
Smiddy WE, Maguire AM, Green WR, Michels RG, de la Cruz Z, Enger C et al. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology 1989; 96: 811–820; discussion 821.
Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC . Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol 1981; 99: 1445–1454.
Jaffe NS . Vitreous traction at the posterior pole of the fundus due to alterations in the vitreous posterior. Trans Am Acad Ophthalmol Otolaryngol 1967; 71: 642–652.
Ripandelli G, Coppe AM, Parisi V, Olzi D, Scassa C, Chiaravalloti A et al. Posterior vitreous detachment and retinal detachment after cataract surgery. Ophthalmology 2007; 114: 692–697.
Acknowledgements
SWK and SEC were involved in design and conduct of study, and analysis, management and interpretation of data; SEC, JHL, YTK and SWL were involved in data collection; SEC helped in preparation of manuscript; and SWK helped in review and approval of manuscript. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chung, S., Lee, JH., Kang, S. et al. Characteristics of epiretinal membranes according to the presence or absence of posterior vitreous detachment. Eye 25, 1341–1346 (2011). https://doi.org/10.1038/eye.2011.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.171