Abstract
In forensic medicine, one-third of the sudden deaths remain unexplained after medico-legal autopsy. A major proportion of these sudden unexplained deaths (SUD) are considered to be caused by inherited cardiac diseases. Sudden cardiac death (SCD) may be the first manifestation of these diseases. The purpose of this study was to explore the yield of next-generation sequencing of genes associated with SCD in a cohort of SUD victims. We investigated 100 genes associated with cardiac diseases in 61 young (1–50 years) SUD cases. DNA was captured with the Haloplex target enrichment system and sequenced using an Illumina MiSeq. The identified genetic variants were evaluated and classified as likely, unknown or unlikely to have a functional effect. The criteria for this classification were based on the literature, databases, conservation and prediction of the effect of the variant. We found that 21 (34%) individuals carried variants with a likely functional effect. Ten (40%) of these variants were located in genes associated with cardiomyopathies and 15 (60%) of the variants in genes associated with cardiac channelopathies. Nineteen individuals carried variants with unknown functional effect. Our findings indicate that broad genetic investigation of SUD victims increases the diagnostic outcome, and the investigation should comprise genes involved in both cardiomyopathies and cardiac channelopathies.
Similar content being viewed by others
Introduction
Sudden cardiac death (SCD) is responsible for a large proportion of sudden, unexpected deaths in young individuals (≤50 years). If the death is unexplained after thorough medico-legal investigation, including autopsy, histopathology and toxicology, the death is referred to as sudden unexplained death (SUD). In Denmark, it has been estimated that 7% of all deaths is due to SCD in the age group 1–35 years.1 Of the SCDs that were autopsied in that study, 29% remained unexplained after medico-legal investigation.1 In the age group 1–49 years, the fraction of SCD cases was 11% of all deaths in Denmark.2 Generally, SUD is considered to be caused by inherited cardiac diseases, especially cardiac channelopathies such as long QT syndrome, Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia, short QT syndrome, progressive cardiac conduction disorder or early repolarization syndrome, that is, cardiac diseases without morphological abnormalities. Gene mutations associated with the development of cardiomyopathies have also been suspected to be involved in SUD, although the mechanisms are still unknown.3, 4, 5, 6 In these cases, SUD may represent an early malignant arrhythmogenic presentation – preceding the development of the morphological cardiomyopathy phenotype. Many studies have been done to investigate the genetics in cases of SUD in the young. Most studies have focused on the cardiac channelopathy-associated genes7, 8, 9, 10, 11 and only a few have investigated if cardiomyopathy-associated gene mutations might also be involved.12, 13, 14
The purpose of this study was to explore the utility of next-generation sequencing (NGS) of a large number of genes associated with both the cardiac channelopathies and cardiomyopathies in a cohort of 61 SUD victims in a forensic setting in Denmark.
Materials and methods
Study population
Between 2009 and 2011, 348 young (1–50 years) deceased individuals, who were assumed to have died from natural causes, were autopsied at the Section of Forensic Pathology, Department of Forensic Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark. Of these, 142 individuals were categorized as SCD, including cases with negative autopsy. In total, 61 individuals had died suddenly, unexpectedly and with no pathogenic findings after thorough macro- and histopathological investigations. Toxicology screening did not show any drug in a toxic level that could explain the death in any of these cases. The remaining 81 cases were examined in earlier studies due to other cardiac causes of death. Twenty individuals were classified as having cardiomyopathies (arrhythmogenic right ventricular cardiomyopathy (ARVC)=14, hypertrophic cardiomyopathy (HCM)=6), 52 individuals as having non-diagnostic structural abnormalities of the heart15 and 9 individuals as sudden unexpected death in infancy.16
Genetic investigation
We have previously described the methods for the genetic investigations in Hertz et al.15 In brief, DNA was extracted from whole blood in 57 of the individuals using the QIAamp DNA Mini Kit (Qiagen, Stockach, Germany). DNA was extracted from fresh frozen muscle from one individual and from fresh frozen spleen tissue from three individuals, using the EZ1 DNA Investigator Kit (Qiagen). The extracted DNA was quantified with the Quantifiler Human DNA Quantification Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol. DNA from two samples was whole-genome amplified using the REPLI-g Midi kit (Qiagen) following the manufacturer’s protocol due to low DNA concentration.
NGS was used to investigate 100 genes previously reported to be associated with inherited cardiac diseases and SCD (Table 1). The genes were selected from the available literature in PubMed and Online Mendelian Inheritance in Man (http://omim.org/).
SureDesign (Agilent Technologies, Santa Clara, CA, USA) was used to design capture probes for the target regions of the exons and UTRs of the 100 genes for the Haloplex Target Enrichment system (Agilent Technologies) with 150 bp read lengths. The design included all coding exons, 25 bp of the adjacent introns and 5′- and 3′-UTR regions of the 100 genes of interest to a total of 2076 regions with a total size of 787 943 bp. Overall, 99.6% of the target was covered >50 ×. Two exons were not covered sufficiently by the sequencing and seven exons were not sequenced due to missing probe designs. Details of the genomic regions not covered sufficiently is found in Supplementary Table S1. Median coverage for amplicons was 950 (range: 206–3065).
The Haloplex Target Enrichment protocol version D.5 was used according to the manufacturer’s instruction. The protocol included the following steps: (1) digestion of 200 ng of genomic DNA with different restriction enzymes in eight tubes and analysis of the fragments using a 2100 Bioanalyzer (Agilent Technologies); (2) hybridization of the digested DNA to Haloplex probes with ends of the probes that were complementary to the fragments of the targets. During hybridization, the fragments were circularized and indexes, sequencing motifs and biotin were incorporated; (3) the target DNA was captured with Haloplex magnetic beads; (4) the nicks in the circularized fragments were closed by ligation; and (5) elution with NaOH, PCR amplification, purification and final elution with Tris-HCl buffer of the captured target DNA was performed. After library build, the amount of DNA was measured using a Qubit Fluorometer 2.0 with the dsDNA HS assay (Invitrogen, Life Technologies Europe BV, Nærum, Denmark). The size distribution of the DNA was analyzed using a 2100 Bioanalyzer and the High Sensitivity DNA kit (Agilent Technologies). All samples were sequenced on a MiSeq (Illumina, San Diego, CA, USA) according to the manufacturer’s recommendations with 150 bp paired-end sequencing using the MiSeq Reagent Kit V2 (300 cycles).
Data analysis and bioinformatics
We have previously described the data analysis in details in Hertz et al.15 In brief, fastq files generated by the MiSeq were used for further alignment and analysis. SureCall (Agilent Technologies) was used for post-base-calling analysis using default settings and their standard algorithm. A complete list of identified variants was given in Variant Caller Format.
Alamut Batch v.1.2.0 and Alamut Visual v.2.5 (Interactive Biosoftware, Rouen, France) were used for the annotation and evaluation of variants as described by Hertz et al.11 The variants were evaluated by (1) an in-house in silico analysis tool developed for determining the likelihood of variant pathogenicity and (2) frequencies of <1% in two reference population databases (the Exome Variant Server (ESP) (http://evs.gs.washington.edu/EVS/) and dbSNP (build 137; http://www.ncbi.nlm.nih.gov/snp/)) and associations with cardiac diseases reported in HGMD.17
The in silico analysis was based on the parameters of conservation (Grantham distance, AlignGVGD class, BLOSUM62 and orthologues), computational prediction (MAPP and SIFT), frequency in the relevant reference population (dbSNP (build 137) and ESP), the location in the genome (distance to nearest splice site) and the coding effect. The available databases and literature were also reviewed for each variant to determine their possible effects.18 Evidence for pathogenicity included null variants (nonsense, frameshift, splice site and initiation codon), known disease-causing amino-acid changes or residues, functional studies, prevalence of the variants in affected individuals with the associated disease, location in exon and/or functional domain with known disease-causing variants, assumed de novo, co-segregation studies and a minor allele frequency below the disease prevalence.18, 19, 20 Given that rare variants are also subject to population stratification,21 we included a local Danish reference population (n=2000) that was whole-exome sequenced, of which one half was comprised of metabolically healthy individuals and the other half was patients with type 2 diabetes.22 The variants were finally classified as likely, unknown or unlikely to have a functional effect by two medical doctors (CLH and MD).
A variant was classified as being of unknown significance, in case of frequency above the disease-prevalence according to available literature, non-conserved amino-acid substitution, multiple observations of the variant in the cohort or lack of significant association with any cardiac disease and nucleotides in the regions, in which the variant was found.
Genes and DNA variants were numbered according to the reference sequences applied in Table 1, using HGVS nomenclature (www.HGVS.org). Variants were submitted to the Leiden Open Variation Database (http://databases.lovd.nl/shared/individuals, individuals IDs: 00064702–00064741).
Coverage was calculated using a python script that calculates coverage for each amplicon using Bedtools coverageBed. The amplicons where all samples had <50 × coverage were extracted to an excel file. The input was BAM files of each sample and a BED file containing all the amplicons from the SureDesign (Agilent Technologies) of the 100 genes.
The software R v.2.11.023 was used to perform the statistical analyses and the scoring of variants in the in silico analysis using a custom script.
Ethical standards
The study was approved by the Committees on Health Research Ethics in the Capital Region of Denmark (H-2-2012-017) and the Danish Data Protection Agency (2011-54-1262).
Results
The study population
We included 61 individuals, who had died suddenly and unexpectedly with no pathogenic findings after thorough medico-legal investigations and were classified as SUD. The median age at the time of death was 39 years (range 1–50) and 43 (70%) were males (Table 2). Post-mortem toxicological screenings were performed in 54 (89%) of the cases of which 16 (30%) were negative. No drug was found in a toxic concentration that could explain the death in any of the cases.
Two deceased individuals (3%) had a family history of cardiac disease. The father of one individual died at the age of 56 years of unspecified cardiac cause. No variant with likely functional effect was found in this individual. Two close relatives of the other individual died during sleep at a young age with no recognized cause. In this individual, two variants with likely functional effects were found (Table 3, ID 7).
In our study, 16 (26%) of the SUD cases had psychiatric diseases according to the available medical records. Seven (13%) and eight (15%) of the deceased used antipsychotics or antidepressants, respectively. According to the available summary of product characteristics, the antipsychotics and antidepressants used by the deceased had a very low or unknown frequency of QTc prolongation. Further, 14 (23%) of the SUD cases suffered from epilepsy and 10 (19%) had one or more antiepileptic drug(s) in the blood (Table 2). None of the drugs used to treat epilepsy had a known frequency of QTc prolongation according to the available summary of product characteristics.
Variants with a likely and unknown functional effect
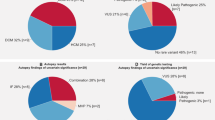
In total, 21 (34%) individuals had 25 genetic variants that were classified as having likely functional effects (Table 3). Ten of these variants were novel. The median age of individuals carrying variants with a likely functional effect was 36 years (range 1–49) and included 11 males (52%). Of the variants with a likely functional effect, 22 were missense mutations and 3 were nonsense mutations (Table 3). Ten (40%) and 15 (60%) variants were located in genes previously having been reported to be associated with cardiomyopathies or cardiac channelopathies, respectively. Seven of the individuals who had variants with likely functional effects also had variants of unknown functional effect. Further, 19 individuals only carried genetic variants of unknown functional effect (data on unknown variants are found in the Supplementary Table S2). In the remaining 21 (34%) individuals, no variant with likely or unknown functional effect was found.
Physical activity at the time of death
Six (10%) of the SUD cases died during the physical activity. Of these, four had a variant with a likely functional effect. A 39-year-old female collapsed while walking and had a variant with a likely functional effect in KCNJ8 (c.1066C>T, p.(R356*)). She had no prior reported symptoms of cardiac disease. Two individuals died while diving: a 46-year-old female, who had one variant with a likely functional effect in MYBPC3 (c.442G>A, p.(G148R)) and another in TNNC1 (c.433G>A, p.(D145N)), and a 35-year-old male with a variant with likely functional effect in MYBPC3 (c.3742G>A, p.(G1248R)). The two divers had not reported any prior symptoms indicating cardiac disease. A 15-year-old male collapsed suddenly during soccer while running. He had one variant with a likely functional effect in CACNB2 (c.641G>C, p.(S214T)) and another in MYBPC3 (c.649A>G, p.(S217G)). He had previously experienced two syncope events during soccer and was under examination for cardiac disease when he died. The ECG showed ventricular extrasystoles, but no diagnosis had been established.
Discussion
We sequenced 100 cardiac disease-associated genes in 61 Danish cases of SUD using NGS. In 21 (34%) of the individuals, one or more variants with a likely functional effect were identified. Of the 21 individuals, 12 had variants with likely functional effects exclusively in genes that have previously been associated with cardiac ion channel diseases. Six individuals had variants with likely functional effects exclusively in genes that have previously been associated with cardiomyopathic diseases. Three individuals had variants with likely functional effects in both channelopathy- and cardiomyopathy-associated genes.
The frequency of variants in cardiomyopathy-associated genes was higher than expected. Cardiomyopathies are often variable in expression and with incomplete penetrance, and the initial phenotypic alterations may not be visible at autopsy or may be considered unspecific or within the normal range. However, variants previously reported to be associated with cardiomyopathies and structural alterations of the heart may give rise to arrhythmia – and in some cases mediated through cardiac channel dysfunctions. This is supported by functional studies of desmosomal genes, previously demonstrated to be associated with ARVC.3, 4, 5, 6 The PKP2 gene was first shown to be associated with ARVC, when Gerull et al24 found 25 variants in PKP2 in 32 ARVC patients. Variants in PKP2 have been found in ~70% of all familial ARVC cases.4, 25 Recently, PKP2 was found to be associated with BrS with no structural abnormalities of the heart.3, 4 Cerrone et al3 found PKP2 variants in 2.5% of the investigated BrS patients. In that study, PKP2 was therefore suggested to play a role in the sodium channel trafficking to the intercalated disk through an unknown mechanism.3 Zhang et al4 found equal numbers of PKP2 variants in ARVC patients and SUD cases with negative autopsy, and concluded that sudden death could be due to arrhythmia via reduced localization of the Nav1.5 channel proteins to the intercalated disk and, hence, increase the distance between the plus end of the microtubules and the N-cadherins midline plaque. Variants in DSP and DSG2, which are also part of the cardiac desmosomal complex, have similarly been found to be associated with Nav1.5 dysfunction in mice and HL-1 cells, respectively.5, 6 Also, sarcomeric gene mutations were present in sudden infant death syndrome (SIDS) cases, in which no structural change was seen. Brion et al26 found multiple, previously described variants in sarcomeric genes in 286 SIDS victims. They suggested that variants in sarcomeric genes could be the cause of death in these infants even though the infants had no structural changes of the heart.
In the present study, we found two-thirds (40 individuals) of the SUD cases had variants with unknown functional effects or no variants with likely or unknown functional effects. These individuals’ cause of death still remain unanswered and raises new questions; are there other genes or regulatory elements (methylation, miRNA, etc) that are only found in SUD cases, and never presents themselves in individuals that are living with a heart disease? It would be of interest to investigate these cases further with whole-genome sequencing and investigate epigenetic factors.
Physical activity at the time of death
Six (10%) of the SUD cases died during the physical activity, including walking, diving, biking or playing soccer. Four of these six individuals had variants with likely functional effects, whereof three were in MYBPC3. A 46-year-old female diver had a MYBPC3 variant (c.442G>A) previously observed in an HCM patient.27 A 35-year-old male diver had a MYBPC3 variant (c.3742G>A) previously observed in another HCM patient28 and classified as probably pathogenic.29 A 15-year-old athlete football player had a MYBPC3 variant (c.649A>G) previously identified in a study, where the variant was found in two SIDS cases and described as possibly pathogenic.26 In another study, the variant was found in a 19-year-old female, who experienced cardiac arrest after running and was subsequently diagnosed with HCM.30 Furthermore, the variant was described by the same study as above by Andreasen et al,29 who found this variant to be of low frequency and possibly pathogenic. The three individuals with MYBPC3 variants with a likely functional effect were all performing moderate to heavy exercise at the time of death.
Sudden unexpected death in psychiatric disease and epilepsy
In the present SUD cohort, 16 (26%) of the SUD cases had psychiatric diseases according to the available medical records (Table 2). One or more variants with a likely functional effect were found in five (31%) of the individuals with a psychiatric disease. The variants were found in the SCN5A, KCNQ1, TTN and LMNA genes. The prevalence of SUD in the young with depression has not been widely studied. It is known that the life expectancy in Denmark in schizophrenic patients is 16–20 years shorter than that of the general population.31 In a recent study from Australia, Sweeting et al32 found that the most prevalent cause of death in schizophrenic patients was cardiovascular disease (23%) and that 11% of the deaths were unexplained after autopsy.
The underlying causes of SCD and SUD in psychiatric diseases are not yet established, but drug-induced arrhythmia has been proposed to be important in depression33 and schizophrenia.34, 35, 36, 37
Furthermore, 14 (23%) of the SUD cases suffered from epilepsy and 10 (19%) had one or more antiepileptic drug(s) in the blood. Sudden unexpected death in epilepsy (SUDEP) is defined as sudden, unexpected, witnessed or unwitnessed, non-traumatic and non-drowning death in patients with epilepsy with or without evidence for seizure with exclusion of documented status epilepticus and when post-mortem examinations do not reveal any structural or toxicological causes of death.38 In line with our findings, a Danish study by Holst et al39 identified 166 SUD cases in the period 2000–2006. Among these, 26 cases were diagnosed as definite SUDEP giving a total percentage of epilepsy cases of 16% in their SUD cohort. SUDEP is suspected to be multifactorial and may relate to autonomic dysfunction, abnormalities in heart rate variability or catecholamine surge, antiepileptic medications and underlying cardiac arrhythmias.40, 41 Risk factors for SUDEP in patients treated with antiepileptic medication include poor patient compliance, abrupt withdrawal, poly-pharmacy and specific antiepileptic drugs. Some antiepileptic drugs have been suspected to be associated with a negative effect on cardiac conduction and may induce sodium channel blockage or dose-dependently suppress the autonomic cardiac modulation.41, 42 In our study, 3 of the 14 SUDEP cases (21%) had one or more variants with likely functional effects. These variants were identified in genes involved in the inwardly-rectifying potassium channel coded by the KCNH2 and in a gene involved in the desmosomal complex (DSP) that is associated with the Nav1.5 channel function. In an Australian study, KCNH2 and SCN5A mutations were found in 13% of the SUDEP cases.43
There are some limitations to this study. First of all, this is a highly selected cohort potentially reducing the generalizability of our findings. To investigate the variants with a likely functional effect further, functional studies and co-segregation analyses in the families should be performed. The genetic findings in this study cannot stand alone to determine the cause of death, as many of the variants identified are novel and need to be investigated further.
In summary, we found that one-third of the deceased individuals with negative forensic autopsy had one or more variants with a likely functional effect in genes associated with cardiomyopathies and cardiac channelopathies. Furthermore, 19 and 21 individuals were found to have only variants of unknown significance or no variants of significance, respectively. Our findings suggest that broad genetic screening using NGS is a useful diagnostic part of the forensic investigation in SUD cases, in which the cause of death cannot be established after routine medico-legal autopsy. As a significant proportion of the variants with a likely functional effect were found in genes previously found to be associated with cardiomyopathies, we suggest that genetic investigations of SUD victims should comprise genes involved in both the structural and non-structural cardiac diseases. Furthermore, the two-thirds of individuals where no variant with likely functional effect was found make us wonder if there are other genes or regulatory elements that are yet to be discovered in these unexplained sudden death cases.
References
Winkel BG, Holst AG, Theilade J et al: Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J 2011; 32: 983–990.
Risgaard B, Winkel BG, Jabbari R et al: Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in Denmark. Circ Arrhythm Electrophysiol 2014; 7: 205–211.
Cerrone M, Lin X, Zhang M et al: Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014; 129: 1092–1103.
Zhang M, Tavora F, Oliveira JB et al: PKP2 mutations in sudden death from arrhythmogenic right ventricular cardiomyopathy (ARVC) and sudden unexpected death with negative autopsy (SUDNA). Circ J 2012; 76: 189–194.
Zhang Q, Deng C, Rao F et al: Silencing of desmoplakin decreases connexin43/Nav1.5 expression and sodium current in HL1 cardiomyocytes. Mol Med Rep 2013; 8: 780–786.
Rizzo S, Lodder EM, Verkerk AO et al: Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012; 95: 409–418.
Skinner JR, Crawford J, Smith W et al: Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm 2011; 8: 412–419.
Winkel BG, Larsen MK, Berge KE et al: The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol 2012; 23: 1092–1098.
Tester DJ, Medeiros-Domingo A, Will ML, Haglund CM, Ackerman MJ : Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 2012; 87: 524–539.
Kauferstein S, Kiehne N, Jenewein T et al: Genetic analysis of sudden unexplained death: a multidisciplinary approach. Forensic Sci Int 2013; 229: 122–127.
Hertz CL, Christiansen SL, Ferrero-Miliani L et al: Next-generation sequencing of 34 genes in sudden unexplained death victims in forensics and in patients with channelopathic cardiac diseases. Int J Legal Med 2015; 129: 793–800.
Narula N, Tester DJ, Paulmichl A, Maleszewski JJ, Ackerman MJ : Post-mortem Whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: a case series. Pediatr Cardiol 2015; 36: 768–778.
Bagnall RD, Das KJ, Duflou J, Semsarian C : Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm 2014; 11: 655–662.
Loporcaro CG, Tester DJ, Maleszewski JJ, Kruisselbrink T, Ackerman MJ : Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next-generation sequencing. Arch Pathol Lab Med 2014; 138: 1083–1089.
Hertz CL, Christiansen SL, Ferrero-Miliani L et al: Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int J Legal Med 2015; 130: 91–102.
Hertz CL, Christiansen SL, Larsen MK et al: Genetic investigations of sudden unexpected deaths in infancy using next-generation sequencing of 100 genes associated with cardiac diseases. Eur J Hum Genet 2015; 24: 817–822.
Stenson PD, Ball EV, Mort M et al: Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 2003; 21: 577–581.
Richards S, Aziz N, Bale S et al: Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–423.
Ng D, Johnston JJ, Teer JK et al: Interpreting secondary cardiac disease variants in an exome cohort. Circ Cardiovasc Genet 2013; 6: 337–346.
Dorschner MO, Amendola LM, Turner EH et al: Actionable, pathogenic incidental findings in 1000 participants’ exomes. Am J Hum Genet 2013; 93: 631–640.
Mathieson I, McVean G : Differential confounding of rare and common variants in spatially structured populations. Nat Genet 2012; 44: 243–246.
Lohmueller KE, Sparso T, Li Q et al: Whole-exome sequencing of 2000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am J Hum Genet 2013; 93: 1072–1086.
R Core Team. R: A language and environment for statistical computing, 2014.
Gerull B, Heuser A, Wichter T et al: Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 2004; 36: 1162–1164.
Christensen AH, Benn M, Tybjaerg-Hansen A, Haunso S, Svendsen JH : Missense variants in plakophilin-2 in arrhythmogenic right ventricular cardiomyopathy patients—disease-causing or innocent bystanders? Cardiology 2010; 115: 148–154.
Brion M, Allegue C, Santori M et al: Sarcomeric gene mutations in sudden infant death syndrome (SIDS). Forensic Sci Int 2012; 219: 278–281.
Zimmerman RS, Cox S, Lakdawala NK et al: A novel custom resequencing array for dilated cardiomyopathy. Genet Med 2010; 12: 268–278.
Morita H, Rehm HL, Menesses A et al: Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med 2008; 358: 1899–1908.
Andreasen C, Nielsen JB, Refsgaard L et al: New population-based exome data are questioning the pathogenicity of previously cardiomyopathy-associated genetic variants. Eur J Hum Genet 2013; 21: 918–928.
Roberts JD, Veinot JP, Rutberg J, Gollob MH : Inherited cardiomyopathies mimicking arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol 2010; 19: 316–320.
Laursen TM, Nordentoft M, Mortensen PB : Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014; 10: 425–448.
Sweeting J, Duflou J, Semsarian C : Postmortem analysis of cardiovascular deaths in schizophrenia: a 10-year review. Schizophr Res 2013; 150: 398–403.
Weeke P, Jensen A, Folke F et al: Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther 2012; 92: 72–79.
Fanoe S, Kristensen D, Fink-Jensen A et al: Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J 2014; 35: 1306–1315.
Straus SM, Bleumink GS, Dieleman JP et al: Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004; 164: 1293–1297.
Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT : Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry 2001; 58: 1161–1167.
Ray WA, Chung CP, Murray KT, Hall K, Stein CM : Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–235.
Nashef L : Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997; 38 (Suppl 11): S6–S8.
Holst AG, Winkel BG, Risgaard B et al: Epilepsy and risk of death and sudden unexpected death in the young: a nationwide study. Epilepsia 2013; 54: 1613–1620.
Partemi S, Vidal MC, Striano P et al: Genetic and forensic implications in epilepsy and cardiac arrhythmias: a case series. Int J Legal Med 2015; 129: 495–504.
Verma A, Kumar A : Sudden unexpected death in epilepsy: some approaches for its prevention and medico-legal consideration. Acta Neurol Belg 2015; 115: 207–212.
Bermeo-Ovalle AC, Kennedy JD, Schuele SU : Cardiac and Autonomic Mechanisms Contributing to SUDEP. J Clin Neurophysiol 2015; 32: 21–29.
Tu E, Bagnall RD, Duflou J, Semsarian C : Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol 2011; 21: 201–208.
Ponting CP : Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem Sci 2000; 25: 48–50.
Burashnikov E, Pfeiffer R, Barajas-Martinez H et al: Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 2010; 7: 1872–1882.
Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L : Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. J Am Coll Cardiol 1999; 34: 823–829.
Van Driest SL, Vasile VC, Ommen SR et al: Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44: 1903–1910.
Tester DJ, Will ML, Haglund CM, Ackerman MJ : Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005; 2: 507–517.
Zareba W, Moss AJ, Sheu G et al: Location of mutation in the KCNQ1 and phenotypic presentation of long QT syndrome. J Cardiovasc Electrophysiol 2003; 14: 1149–1153.
van Tintelen JP, Entius MM, Bhuiyan ZA et al: Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 2006; 113: 1650–1658.
Shy D, Gillet L, Ogrodnik J et al: PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation 2014; 130: 147–160.
Kapplinger JD, Tester DJ, Salisbury BA et al: Spectrum and prevalence of mutations from the first 2500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 2009; 6: 1297–1303.
Acknowledgements
We thank Francisc-Raul Kantor and Carina Grøntved Jønck for bioinformatics support, and Eva Tonnesen for technical support in the laboratory. For the screening of variants among Danish controls, we thank LuCamp, The Lundbeck Foundation Centre for Applied Medical Genomics in Personalized Disease Prediction, Prevention and Care (www.lucamp.org), and the Novo Nordisk Foundation Center for Basic Metabolic Research, which is an independent Research Center at the University of Copenhagen partially supported by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk). This work was supported by Ellen and Aage Andersen’s Foundation and The AP Møller Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Christiansen, S., Hertz, C., Ferrero-Miliani, L. et al. Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur J Hum Genet 24, 1797–1802 (2016). https://doi.org/10.1038/ejhg.2016.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2016.118
This article is cited by
-
Whole exome sequencing with a focus on cardiac disease-associated genes in families of sudden unexplained deaths in Yunnan, southwest of China
BMC Genomics (2023)
-
Historical perspective and recent progress in cardiac ion channelopathies research and clinical practice in Hong Kong
International Journal of Arrhythmia (2023)
-
Genetics and genomics of arrhythmic risk: current and future strategies to prevent sudden cardiac death
Nature Reviews Cardiology (2021)
-
Postmortale molekulargenetische Untersuchungen (molekulare Autopsie) bei kardiovaskulären und bei ungeklärten Todesfällen
Der Kardiologe (2021)
-
Genetic investigations of 100 inherited cardiac disease-related genes in deceased individuals with schizophrenia
International Journal of Legal Medicine (2021)