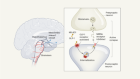
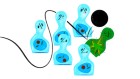
Researchers adapted a peptide similar to the one used in the obesity drug Wegovy to have an even more potent weight-loss response in mice.Credit: Lise Aaserud/NTB/Alamy
With obesity drugs now helping people to slim down, researchers are working to capitalize on their popularity by bulking up the weight-loss drug pipeline. The latest contender takes a Trojan horse approach — hiding a small molecule in a gut-hormone-mimicking peptide already used in obesity drugs — to strike a double blow to the brain cells that control appetite.
Obesity drugs aren’t always forever. What happens when you quit?
The new work, which demonstrated the effects of this drug candidate in mice and rats, was published today in Nature1.
“It’s a strong paper,” says Daniel Drucker, an endocrinologist at Mount Sinai Hospital in Toronto, Canada, who helped to unravel the role of gut hormones such as GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) in obesity. The blockbuster weight-loss drugs semaglutide (Wegovy) and tirzepatide (Zepbound) act by mimicking these hormones, binding to their receptors on neurons in the brain that control hunger pangs. These drugs can help people to lose 15–20% of their body weight. And it could be possible to eke even more activity from these hormone mimics by fusing them to other drugs, the new study suggests.
“Very high marks for the novelty” of the research, says Drucker, who was not involved and consults for the pharmaceutical industry. “Let’s hope that we’ll see some proof of concept in the clinic”, when the approach is tested in humans.
Trojan therapeutics
The drug contender takes aim at both the GLP-1 receptor and the NMDA receptor, an ion channel found on cells in the brain that was linked to obesity in 20152. At the time, small molecules that blocked the NMDA receptor seemed like a non-starter for obesity-drug developers, because this type of compound, which includes the party drug and antidepressant ketamine, is riddled with harmful side effects.
Obesity drugs have another superpower: taming inflammation
But Christoffer Clemmensen, a metabolism specialist at the University of Copenhagen, saw a path forwards. He speculated that it might be possible to sidestep the safety risks by fusing an NMDA-receptor blocker to a gut-hormone mimic that acts only on the neurons that regulate appetite.
To make this a reality, Clemmensen and his colleagues attached a peptide that looks like the GLP-1 hormone to a small molecule, dizocilpine (also called MK-801), that blocks the NMDA receptor. Dizocilpine was discovered in the 1980s by researchers at the pharmaceutical firm Merck & Co., based in Rahway, New Jersey, but then abandoned. Clemmensen and the team saw that, in mice and rats, GLP-1-loving neurons in the brain would take up this peptide–drug conjugate, and then cut the dizocilpine payload loose to block the NMDA receptor. (Some members of the team work at Novo Nordisk, which makes semaglutide, although Clemmensen says this was an academic collaboration and not a commercial one.)
“This is a really creative way to optimize for weight loss,” says Darleen Sandoval, a physiologist at the University of Colorado in Aurora. “The big picture here is how far we have come in terms of being able to target the brain to treat obesity,” adds Sandoval, who co-authored an accompanying commentary about the study in Nature3.
Treating mice with dizocilpine alone caused side effects such as overheating and excess movement. The peptide–drug conjugate was safer, and it offered similar weight-loss benefits to treating mice with semaglutide alone. Where the conjugate shone was in mice pre-dosed with semaglutide: once the animals reached a weight-loss plateau with that drug, giving them the conjugate as an add-on treatment drove their body mass down further.
“It is competitive with the current best therapies on the market,” says Clemmensen. “Possibly, we can outperform these.”
To the clinic
As a next step, Clemmensen and some colleagues have co-founded Ousia Pharma, based in Copenhagen, to advance a related drug candidate into clinical trials. This potential therapeutic, called OP-216, has the added benefit of also mimicking GIP in addition to GLP-1, Clemmensen says. “We could be in the clinic in 2025,” he adds.
Beyond Ozempic: brand-new obesity drugs will be cheaper and more effective
The success of the current crop of obesity drugs has set a high bar for next-generation therapeutics. But “there’s definitely room for more drugs and targets”, says Ruth Loos, an obesity geneticist at the University of Copenhagen who co-led the 2015 genetics study that linked the NMDA receptor to obesity2. Not everyone sheds weight using the currently available options. And gut-hormone mimics need to be taken continuously to have an effect.
Loos, who has also consulted for the pharmaceutical industry, was not involved in the development of the latest peptide–drug conjugate, but hopes it will encourage others to look for innovative ways to treat obesity. Dozens of weight-loss drugs are already in the clinic — many targeting GLP-1 and GIP — and drug developers are on the lookout for up-and-coming agents, especially given that the weight-loss drug market is forecast to be worth up to US$100 billion by 2030.
By 2035, over half of adults worldwide are predicted to be obese. Treating them with obesity drugs could confer wider health advantages, such as cardiovascular and anti-inflammatory benefits. Trials of these drugs are also under way for kidney disease, Parkinson’s and Alzheimer’s disease and addiction-related behaviours such as drinking and smoking.
“Not all these trials are going to be successful,” Drucker says. But enough might pan out to reshape the therapeutic landscape, he adds. “It’s going to be fascinating to watch.”
“When I started working on obesity in 2013, there was no interest in it,” Clemmensen says. Right now, he adds, all the activity is a little bit wild.

 Obesity drugs aren’t always forever. What happens when you quit?
Obesity drugs aren’t always forever. What happens when you quit?
 Obesity drugs have another superpower: taming inflammation
Obesity drugs have another superpower: taming inflammation
 Beyond Ozempic: brand-new obesity drugs will be cheaper and more effective
Beyond Ozempic: brand-new obesity drugs will be cheaper and more effective
 Game-changing obesity drugs go mainstream: what scientists are learning
Game-changing obesity drugs go mainstream: what scientists are learning
 The ‘breakthrough’ obesity drugs that have stunned researchers
The ‘breakthrough’ obesity drugs that have stunned researchers