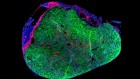
- NEWS AND VIEWS
Human embryonic stem cells get organized
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 558, 35-36 (2018)
doi: https://doi.org/10.1038/d41586-018-05115-y
References
Spemann, H. & Mangold, H. Wilhelm Roux Arch. Entw. Mech. Org. 100, 599–638 (1924).
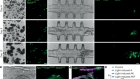
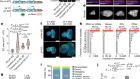
Martyn, I., Kanno, T. Y., Ruzo, A., Siggia, E. D. & Brivanlou, A. H. Nature 558, 132–135 (2018).
Arias, A. M. & Steventon, B. Development 145, dev159525 (2018).
Beddington, R. S. P. Development 120, 613–620 (1994).
Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. & Brivanlou, A. H. Nature Meth. 11, 847–854 (2014).
Crease, D. J., Dyson, S. & Gurdon, J. B. Proc. Natl Acad. Sci. USA 95, 4398–4403 (1998).
Gritsman, K., Talbot, W. S. & Schier, A. F. Development 127, 921–932 (2000).
Cho, K. W. Y., Blumberg, B., Steinbeisser, H. & De Robertis, E. M. Cell 67, 1111–1120 (1991).

 Read the paper: Self-organization of a human organizer by combined Wnt and Nodal signalling
Read the paper: Self-organization of a human organizer by combined Wnt and Nodal signalling
 Implantation barrier overcome
Implantation barrier overcome
 Mechanics in the embryo
Mechanics in the embryo