- NEWS AND VIEWS
Rule-breaking perovskites
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 553, 163-164 (2018)
doi: https://doi.org/10.1038/d41586-018-00012-w
References
Becker, M. A. et al. Nature 553, 189–193 (2018).
Pope, M. & Swenberg, C. E. Electronic Processes in Organic Crystals (Oxford Univ. Press, 1999).
Reineke, S., Thomschke, M., Lüssem, B. & Leo, K. Rev. Mod. Phys. 85, 1245–1293 (2013).
Demtröder, W. Atoms, Molecules and Photons (Springer, 2010).
Rainò, G. et al. ACS Nano 10, 2485–2490 (2016).
Stoumpos, C. C. & Kanatzidis, M. G. Acc. Chem. Res. 48, 2791–2802 (2015).
Correa-Baena, J.-P. et al. Science 358, 739–744 (2017).
Rau, U. Phys. Rev. B 76, 085303 (2007).
Eperon, G. E., Hörantner, M. T. & Snaith, H. J. Nature Rev. Chem. 1, 0095 (2017).
Colella, S., Mazzeo, M., Rizzo, A., Gigli, G. & Listorti, A. J. Phys. Chem. Lett. 7, 4322–4334 (2016).

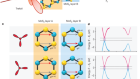
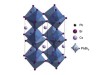 Read the paper: Bright triplet excitons in caesium lead halide perovskites
Read the paper: Bright triplet excitons in caesium lead halide perovskites
 Twenty-five years of low-cost solar cells
Twenty-five years of low-cost solar cells
 Clockwork at the atomic scale
Clockwork at the atomic scale