Abstract
Nanostructured semiconductors emit light from electronic states known as excitons1. For organic materials, Hund’s rules2 state that the lowest-energy exciton is a poorly emitting triplet state. For inorganic semiconductors, similar rules3 predict an analogue of this triplet state known as the ‘dark exciton’4. Because dark excitons release photons slowly, hindering emission from inorganic nanostructures, materials that disobey these rules have been sought. However, despite considerable experimental and theoretical efforts, no inorganic semiconductors have been identified in which the lowest exciton is bright. Here we show that the lowest exciton in caesium lead halide perovskites (CsPbX3, with X = Cl, Br or I) involves a highly emissive triplet state. We first use an effective-mass model and group theory to demonstrate the possibility of such a state existing, which can occur when the strong spin–orbit coupling in the conduction band of a perovskite is combined with the Rashba effect5,6,7,8,9,10. We then apply our model to CsPbX3 nanocrystals11, and measure size- and composition-dependent fluorescence at the single-nanocrystal level. The bright triplet character of the lowest exciton explains the anomalous photon-emission rates of these materials, which emit about 20 and 1,000 times faster12 than any other semiconductor nanocrystal at room13,14,15,16 and cryogenic4 temperatures, respectively. The existence of this bright triplet exciton is further confirmed by analysis of the fine structure in low-temperature fluorescence spectra. For semiconductor nanocrystals, which are already used in lighting17, lasers18 and displays19, these excitons could lead to materials with brighter emission. More generally, our results provide criteria for identifying other semiconductors that exhibit bright excitons, with potential implications for optoelectronic devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Scholes, G. D. & Rumbles, G. Excitons in nanoscale systems. Nat. Mater. 5, 683–696 (2006)
Hund, F. Concerning the interpretation of complex spectra, especially the elements scandium to nickel. Z. Phys. 33, 345–371 (1925)
Onodera, Y. & Toyozawa, Y. Excitons in alkali halides. J. Phys. Soc. Jpn 22, 833–844 (1967)
Nirmal, M. et al. Observation of the “dark exciton” in CdSe quantum dots. Phys. Rev. Lett. 75, 3728–3731 (1995)
Bychkov, Yu. A. & Rashba, E. I. Oscillatory effects and the magnetic susceptibility of carriers in inversion layers. J. Phys. C 17, 6039–6045 (1984)
Kim, M., Im, J., Freeman, A. J., Ihm, J. & Jin, H. Switchable S=1/2 and J=1/2 Rashba bands in ferroelectric halide perovskites. Proc. Natl Acad. Sci. USA 111, 6900–6904 (2014)
Zheng, F., Tan, L. Z., Liu, S. & Rappe, A. M. Rashba spin–orbit coupling enhanced carrier lifetime in CH3NH3PbI3. Nano Lett. 15, 7794–7800 (2015)
Kepenekian, M. et al. Rashba and Dresselhaus effects in hybrid organic–inorganic perovskites: from basics to devices. ACS Nano 9, 11557–11567 (2015)
Mosconi, E., Etienne, T. & De Angelis, F. Rashba band splitting in organohalide lead perovskites: bulk and surface effects. J. Phys. Chem. Lett. 8, 2247–2252 (2017)
Isarov, M. et al. Rashba effect in a single colloidal CsPbBr3 perovskite nanocrystal detected by magneto-optical measurements. Nano Lett. 17, 5020–5026 (2017)
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015)
Rainò, G. et al. Single cesium lead halide perovskite nanocrystals at low temperature: fast single-photon emission, reduced blinking, and exciton fine structure. ACS Nano 10, 2485–2490 (2016)
Crooker, S. A., Barrick, T., Hollingsworth, J. A. & Klimov, V. I. Multiple temperature regimes of radiative decay in CdSe nanocrystal quantum dots: Intrinsic limits to the dark-exciton lifetime. Appl. Phys. Lett. 82, 2793–2795 (2003)
Wuister, S. F., van Houselt, A., de Mello Donegá, C., Vanmaekelbergh, D. & Meijerink, A. Temperature antiquenching of the luminescence from capped CdSe quantum dots. Angew. Chem. Int. Ed. 43, 3029–3033 (2004)
Du, H. et al. Optical properties of colloidal PbSe nanocrystals. Nano Lett. 2, 1321–1324 (2002)
Bischof, T. S., Correa, R. E., Rosenberg, D., Dauler, E. A. & Bawendi, M. G. Measurement of emission lifetime dynamics and biexciton emission quantum yield of individual InAs colloidal nanocrystals. Nano Lett. 14, 6787–6791 (2014)
Shirasaki, Y., Supran, G. J., Bawendi, M. G. & Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photon. 7, 13–23 (2013)
Dang, C. et al. Red, green and blue lasing enabled by single-exciton gain in colloidal quantum dot films. Nat. Nanotechnol. 7, 335–339 (2012)
Kim, T. H. et al. Full-colour quantum dot displays fabricated by transfer printing. Nat. Photon. 5, 176–182 (2011)
Bir, G. L. & Pikus, G. E. Symmetry and Strain-Induced Effects in Semiconductors Ch. 23 (Wiley, 1974)
Even, J., Pedesseau, L., Jancu, J.-M. & Katan, C. Importance of spin–orbit coupling in hybrid organic/inorganic perovskites for photovoltaic applications. J. Phys. Chem. Lett. 4, 2999–3005 (2013)
Kane, E. O. in Semiconductors and Semimetals Vol. 1 (eds Willardson, R. K. & Beer, A. C. ) 75–100 (Academic Press, 1966)
Koster, G. F., Dimmock, J. O., Wheeler, R. G. & Statz, H. Properties of the Thirty-Two Point Groups (MIT Press, 1963)
Yaffe, O. et al. Local polar fluctuations in lead halide perovskite crystals. Phys. Rev. Lett. 118, 136001 (2017)
Galland, C. et al. Two types of luminescence blinking revealed by spectroelectrochemistry of single quantum dots. Nature 479, 203–207 (2011)
Cottingham, P. & Brutchey, R. L. On the crystal structure of colloidally prepared CsPbBr3 quantum dots. Chem. Commun. 52, 5246–5249 (2016)
Rashba, E. I. & Gurgenishvili, G. E. Edge absorption theory in semiconductors. Sov. Phys. Solid State 4, 759–760 (1962)
Dery, H. & Song, Y. Polarization analysis of excitons in monolayer and bilayer transition-metal dichalcogenides. Phys. Rev. B 92, 125431 (2015)
Fu, M. et al. Neutral and charged exciton fine structure in single lead halide perovskite nanocrystals revealed by magneto-optical spectroscopy. Nano Lett. 17, 2895–2901 (2017)
Rodina, A. V. & Efros, Al. L. Effect of dielectric confinement on optical properties of colloidal nanostructures. J. Exp. Theor. Phys. 122, 554–566 (2016)
Yakunin, S. et al. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 6, 8056 (2015)
Smyder, J. A. et al. The influence of continuous vs. pulsed laser excitation on single quantum dot photophysics. Phys. Chem. Chem. Phys. 16, 25723–25728 (2014)
Mehl, M. J. et al. The AFLOW library of crystallographic prototypes: part 1. Comput. Mater. Sci. 136 (Suppl.), S1–S828 (2017)
Kresse, G. Ab Initio Molekular Dynamik für Flüssige Metalle. PhD thesis, Technische Universität Wien (1993)
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993)
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994)
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994)
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008); erratum 102, 039902 (2009)
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006)
Acknowledgements
We thank F. Krieg for providing large CsPbBr3 nanocrystals, S. Yakunin and J. Jagielski for assistance with absolute quantum-yield measurements, and E. Ivchenko, M. Glazov and E. Rashba for discussions. M.A.B., G.R., T.S., M.V.K. and R.F.M. acknowledge the European Union’s Horizon-2020 programme through the Marie-Skłodowska Curie ITN network PHONSI (H2020-MSCA-ITN-642656) and the Swiss State Secretariat for Education Research and Innovation (SERI). J.G.M., S.G.L., N.B., J.L.L. and Al.L.E. acknowledge support from the US Office of Naval Research (ONR) through the core funding of the Naval Research Laboratory. R.V. was funded by ONR grant N0001416WX01849. A.S. acknowledges support from the Center for Advanced Solar Photophysics (CASP), an Energy Frontier Research Center (EFRC) funded by BES, OS, US DOE. D.J.N. and M.V.K. acknowledge partial financial support from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC grant agreement number 339905 (QuaDoPS Advanced Grant) and number 306733 (NANOSOLID Starting Grant), respectively.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks A. Meijerink, E. Rabani and M. Saba for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
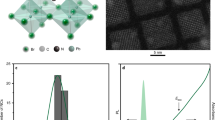
Extended Data Figure 1 Electronic structure for CsPbCl3 and CsPbI3 perovskites.
a, Calculated band structure of cubic perovskite CsPbCl3. b, Calculated band structure of cubic perovskite CsPbI3. See Methods for details about the calculations.
Extended Data Figure 2 Measurements to estimate the low-temperature quantum yield for our CsPbBr2Cl nanocrystals.
a, b, Photoluminescence spectra (a) and decays (b) for CsPbBr2Cl nanocrystals (L = 14 ± 1 nm) embedded in a PMMA film at 295 K (red) and 5 K (black). Data for the two temperatures are plotted on the same intensity scale. For the same sample, a calibrated integrating sphere was used to measure the photoluminescence quantum yield at 295 K (43% ± 1%). To obtain the quantum yield at 5 K, the photoluminescence and optical absorption for several spots at 295 K and 5 K under constant weak excitation (at 3.06 eV) were measured. The photoluminescence increased substantially, as seen in both the spectra and decay signal, whereas the absorption stayed nearly constant (data not shown). From these results, the quantum yield at 5 K was estimated to be 88% ± 14%. The photoluminescence decays in b are plotted on both a linear and a logarithmic (inset) intensity scale, with decay times of 1.60 ns (295 K) and 0.23 ns (5 K). The decrease in decay time at low temperature is clearly accompanied by an increase in the total emitted intensity (area under the decay traces).
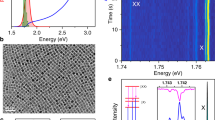
Extended Data Figure 3 Exciton and trion emission from an individual CsPbBr2Cl nanocrystal.
a, Photoluminescence spectrum of a single CsPbBr2Cl nanocrystal, showing exciton peaks at 2.5158 eV (red) and 2.5175 eV (blue) and a trion peak (black) that is redshifted by 15–17 meV. The targeted temperature was 5 K (see Fig. 3 caption). b, Polarization properties of the exciton (left) and trion (right) emission peaks. The normalized area of a Lorentzian-peak fit for the two exciton peaks (red and blue) and the trion peak (black) are shown as a function of the linear polarizer angle (placed in front of the spectrograph). Both exciton peaks show a dominantly linear polarization, with the main axis indicated by the blue and red lines. The trion emission is unpolarized. See Supplementary Section 4 for further discussion.
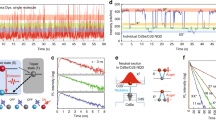
Extended Data Figure 4 Composition-dependent ensemble photoluminescence decay measurements of lead halide perovskite nanocrystals.
a, Typical streak-camera measurement of the photoluminescence from an ensemble of CsPbBr2Cl nanocrystals at 5 K. In this example, the nanocrystals have L = 14 nm. The emission peak is centred at 2.51 eV, and the exponential decay time is 210 ps, as extracted by summing over all energies, which is in good agreement with the results for single CsPbBr2Cl nanocrystals of the same size. The ensemble decay spectrum is slightly asymmetric (being faster at higher energies), which might originate from the activation of an energy-transfer process from smaller to larger nanocrystals. To account for this effect, we considered only the long component of the decay curve. b, Photoluminescence lifetimes at 5 K extracted for ensemble samples of nanocrystals (NCs) of various compositions and sizes (as labelled). The ensemble data (solid circles) are compared with single-nanocrystal measurements (open circles). The good agreement between the two datasets is further evidence that the measured single-nanocrystal photoluminescence decays are due to fast exciton radiative lifetimes and not to trions, because the ensemble data are acquired at very low excitation power, at which photo-generated charging is not observed.
Extended Data Figure 5 Calculation of the interior electric field in cube-shaped nanocrystals.
a, Line plot of the electric potential φ along the centre line between the capacitor plates (see inset and Supplementary Information section 3.B). b, Line plot of the normalized electric-field magnitude  along the centre line between the capacitor plates (see inset).
along the centre line between the capacitor plates (see inset).
Extended Data Figure 6 Contour plots of normalized electric-field magnitude across a cube-shaped nanocrystal.
a–d, Contour plots of  for four different ratios (4, 6, 8 and 10, respectively) of the dielectric constant inside the nanocrystal (εin) and of the surrounding medium (εout) (see Supplementary Information section 3.B). The plots depict the x–z mid-plane of the cube and are valid for the symmetry-equivalent y–z mid-plane. e, Contour plot of
for four different ratios (4, 6, 8 and 10, respectively) of the dielectric constant inside the nanocrystal (εin) and of the surrounding medium (εout) (see Supplementary Information section 3.B). The plots depict the x–z mid-plane of the cube and are valid for the symmetry-equivalent y–z mid-plane. e, Contour plot of  on the x–z mid-plane of the cube for εin/εout = 9. The
on the x–z mid-plane of the cube for εin/εout = 9. The  distribution on the y–z mid-plane is identical. In all panels, the z direction is vertical and the perturbations near the corners of the plots are artefacts of the interpolation resolution used by the software that we used to construct them.
distribution on the y–z mid-plane is identical. In all panels, the z direction is vertical and the perturbations near the corners of the plots are artefacts of the interpolation resolution used by the software that we used to construct them.
Extended Data Figure 7 Extraction of the Kane energy Ep for the lead halide perovskites.
The Kane energy, defined according to equation (5), can be extracted for CsPbCl3, CsPbBr3 and CsPbI3 from the band structures presented in Fig. 1b and Extended Data Fig. 1 near the band edges. The slope of the red line is used, according to a procedure described in Supplementary Information section 1.B.
Extended Data Figure 8 Variational calculations related to the determination of the exciton radiative lifetime in cube-shaped nanocrystals within the intermediate-confinement regime.
a, b, Dimensionless electron–hole correlation constant (b = βL, where β is the value of the variational parameter that minimizes the energy; a) and the square modulus of the ratio of I‖ for intermediate and strong confinement (b) as a function of the size of the nanocrystal relative to the Bohr radius of an electron (L/ae), for the three materials studied; me and mh are the electron and hole effective masses, respectively. The inset in b shows the square modulus of I‖ in the strong-confinement regime for several different dielectric constants, εin/εout. See Supplementary Information section 3.D for details.
Extended Data Figure 9 Representative two-peak spectra for individual CsPbBr2Cl nanocrystals.
a–i, Photoluminescence spectra of different single nanocrystals that exhibit two emission peaks at a targeted temperature of 5 K (see Fig. 3 caption). Each spectrum was recorded with a linear polarizer in the detection path. Therefore, the relative intensities displayed cannot be used to determine the relative (potentially thermal) population within the fine-structure multiplet. The linear polarizer was used here because it can be rotated to resolve all spectral features. Without the polarizer, the low-energy peak typically dominates in intensity.
Extended Data Figure 10 Representative three-peak spectra for individual CsPbBr2Cl nanocrystals.
Details as for Extended Data Fig. 9, but for nanocrystals that exhibit three emission peaks.
Extended Data Figure 11 Predicted exciton spectra and polarization properties for individual perovskite nanocrystals.
The plots show the expected exciton fine structure in photoluminescence spectra from three orthogonal dipoles of the lowest-energy exciton. The dipoles are oriented along the orthorhombic symmetry axes. The insets show the emission probability for the dipoles as a function of the polarization angle. a–d, Expected fine structure for observation in the [010], [001], [011] and [312] directions with respect to the orthorhombic symmetry axes, respectively. The temperature effect on the population of the sublevels is not considered (that is, the populations of the sublevels are assumed to be equal). e–h, As in a–d, but taking the temperature effect on the population of the sublevels into consideration. The temperature is assumed to be comparable to the fine-structure splitting: kBT ≈ Δ1 = Δ2, where kB is the Boltzmann constant and T is temperature. See Supplementary Information section 4 for further details.
Supplementary information
Supplementary Information
This file contains supplementary text S1-S5. (PDF 1240 kb)
Rights and permissions
About this article
Cite this article
Becker, M., Vaxenburg, R., Nedelcu, G. et al. Bright triplet excitons in caesium lead halide perovskites. Nature 553, 189–193 (2018). https://doi.org/10.1038/nature25147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25147
This article is cited by
-
Engineering colloidal semiconductor nanocrystals for quantum information processing
Nature Nanotechnology (2024)
-
Single-photon superradiance in individual caesium lead halide quantum dots
Nature (2024)
-
Single quantum dot spectroscopy for exciton dynamics
Nano Research (2024)
-
Direct linearly polarized electroluminescence from perovskite nanoplatelet superlattices
Nature Photonics (2024)
-
Optical anisotropy of CsPbBr3 perovskite nanoplatelets
Nano Convergence (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.