Abstract
Mixed lineage kinase 3 (MLK3) is a mitogen-activated protein kinase kinase kinase that is activated by tumor necrosis factor-α (TNF-α) and specifically activates c-Jun N-terminal kinase (JNK) on TNF-α stimulation. The mechanism by which TNF-α activates MLK3 is still not known. TNF receptor-associated factors (TRAFs) are adapter molecules that are recruited to cytoplasmic end of TNF receptor and mediate the downstream signaling, including activation of JNK. Here, we report that MLK3 associates with TRAF2, TRAF5 and TRAF6; however only TRAF2 can significantly induce the kinase activity of MLK3. The interaction domain of TRAF2 maps to the TRAF domain and for MLK3 to its C-terminal half (amino acids 511-847). Endogenous TRAF2 and MLK3 associate with each other in response to TNF-α treatment in a time-dependent manner. The association between MLK3 and TRAF2 mediates MLK3 activation and competition with the TRAF2 deletion mutant that binds to MLK3 attenuates MLK3 kinase activity in a dose-dependent manner, on TNF-α treatment. Furthermore the downstream target of MLK3, JNK was activated by TNF-α in a TRAF2-dependent manner. Hence, our data show that the direct interaction between TRAF2 and MLK3 is required for TNF-α-induced activation of MLK3 and its downstream target, JNK.
Similar content being viewed by others
Introduction
Mixed lineage kinase 3 (MLK3) is a mitogen-activated protein kinase kinase kinase (MAPKKK) and belongs to MLK family 1. The MLK family members are characterized by the presence of signature sequences of serine/threonine (Ser/Thr) and tyrosine kinases within their catalytic domain. We and others have shown that some of the MLK members, including MLK3 2, MLK2 3 and DLK 4, possess the functional Ser/Thr kinase activities, however, the tyrosine kinase activity of any MLK family member is still not known. Structurally, MLK family members are highly conserved in their catalytic domain but they diverge in the C-terminal domain, suggesting that probably each family member might be regulated differentially by different ligands. Although the specific ligands for all MLK family members are not yet known, we reported earlier that MLK3 can be activated by tumor necrosis factor-α (TNF-α) 5. TNF-α was also able to activate c-Jun N-terminal kinase (JNK), the downstream target of MLK3, specifically in an MLK3-dependent manner, whereas the activation of other two MAPKs, p38 and ERK, was not mediated through MLK3 5. Although our study showed that TNF-α is a ligand of MLK3 5, the mechanism by which TNF-α activates MLK3 was not understood.
Tumor necrosis factor-α is a pleiotropic cytokine that renders cellular effects by binding to its cognate receptors, TNFR1 and TNFR2. It has been suggested that on encounter with trimeric TNF-α, the TNFRs undergo trimerization, which leads to intracellular signaling 6, 7. Recently, it has been reported that TNFR proteins self-assemble in the absence of ligand, undergoing conformational changes on ligand engagement that lead to downstream signaling 8. Upon conformational changes in the TNFRs, the cytoplasmic ends of TNFRs bind to specific adapter proteins that ultimately transmit the signals to downstream targets 9. Because TNFR proteins lack intrinsic enzymatic activity, the binding of adapter proteins to the cytoplasmic end of the receptor was shown to be essential for TNF-α-mediated signaling 9. One of the protein families that bind to both the TNFRs is TRAFs that have been shown to activate JNK and NF-κB pathways on TNF-α stimulation 10.
Till date, six members of the TRAF family have been described 11, 12, 13. All the TRAFs contain C-terminal TRAF domain that mediates the interaction with TNF receptors and hetero- or homodimerization among the TRAF family members 11. In addition to the TRAF domain, the N terminus of the TRAF proteins, with the exception of TRAF1, contains RING domain and multiple zinc-finger structures; those are essential for their effector functions 9, 12. The TRAF family not only mediates TNFR family-mediated signaling but may also mediate the downstream signaling of other receptors such as the interleukin-1 receptor (IL-1R) 9. Upon ligand engagement by IL-1R, the cytoplasmic end of the receptor recruits a protein complex, including IRAK that binds with TRAF6 and activates JNK, p38, ERK and Src tyrosine kinase 13, 14, 15, 16. Among the six known TRAF proteins, TRAF2, TRAF5 and TRAF6 are reported to activate JNK 17, the downstream target of MLK3 2 and other MLKs 1. It is also reported that TRAF proteins activate the MAPK cascades at the level of MAP3K and MAP4K, including ASK1 18, 19, TAK1 20, MEKK1 21, NIK 22 and GCK 23. Whether the TRAF proteins also recruit MLK3 for TNFR-mediated activation of downstream JNK is yet to be determined.
In this report, we show that although TRAF2, TRAF5 and TRAF6 can all bind to MLK3, only TRAF2 can activate the MLK3 kinase activity. The interaction between TRAF2 and MLK3 was mediated through the TRAF domain of TRAF2, and the C-terminal domain of MLK3. The endogenous TRAF2 and MLK3 also associate with each other, in response to TNF-α treatment, and TRAF2-associated MLK3 possessed significantly higher kinase activity compared to total MLK3 pool. The association between TRAF2 and MLK3 was essential for TNF-α-induced MLK3 activation, as competition with the TRAF domain of TRAF2 was able to attenuate MLK3 activation in a dose-dependent manner. We also show that TRAF2 was essential for TNF-α-induced JNK activation in an MLK3-dependent manner. Together, these data show involvement of TRAF2 in TNF-α-induced MLK3 activation, and its downstream signaling to the JNK pathway.
Results
MLK3 and TRAF proteins associate in vivo
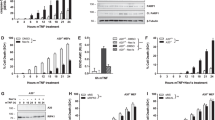
To determine whether TRAF proteins have any role in the activation of MLK3 through TNFRs, we first attempted to identify the TRAF protein(s) that binds with ectopically expressed MLK3. The human embryonic kidney 293 (HEK-293) cells were transiently transfected with equal amounts of glutathione S-transferase (GST)-tagged MLK3 along with either Flag-tagged or Myc-tagged TRAF expression vectors, as indicated (Figure 1). The TRAF proteins were immunoprecipitated either by anti-FLAG (for TRAF2, 5 and 6) or by anti-Myc (for TRAF1 and 3) antibodies and any associated recombinant GST-tagged MLK3 was detected by anti-GST antibody. Our results showed that TRAF2, 5 and 6 are associated with ectopically expressed MLK3, however TRAF1 and TRAF3 failed to co-immunoprecipitate with MLK3 (Figure 1). Taken together, these results indicate that MLK3 associates with TRAF2, 5 and 6 under overexpressed conditions.
MLK3 associates with TRAF proteins. HEK-293 cells were transiently transfected with GST epitope-tagged MLK3 along with either Flag or Myc epitope-tagged TRAF expression vectors, as indicated. The TRAF proteins were immunoprecipitated using antibodies against the specific epitopes and any associated MLK3 was detected by using anti-GST antibody. The expression levels of MLK3 (middle panel) and TRAF proteins (lower two panels) were detected using antibodies against specific epitopes, as indicated.
TRAF2 regulates TNF-α-induced MLK3 kinase activity
Interaction between TRAF2 and some MAP3K members, such as ASK1 18, 19, TAK1 20 and MEKK1 21, has been shown to induce their kinase activities. We examined which of the TRAF proteins (i.e., TRAF2, 5 and 6) that showed interaction with MLK3 (Figure 1) in fact can activate MLK3 kinase activity. HEK-293 cells were transiently transfected with a constant amount of MLK3 and the three TRAF expression vectors, as shown in Figure 2. As controls, some cells were also transfected with TRAF expression vectors alone, to rule out the contribution of any contaminating kinase during the kinase assay. Recombinant MLK3 was precipitated by glutathione sepharose (GSH) pull down and subjected to kinase assay using the MLK3-specific substrate, SEK1 (K-R) protein, expressed in bacteria. MLK3 was approximately 3.5-fold more active in the cells where TRAF2 was transfected (Figure 2A, compare lane 2 with 4), whereas MLK3 was not significantly activated in cells that were transfected with TRAF5 and TRAF6 (Figure 2A). The expression levels of MLK3 and TRAFs were almost equal in all our transfection experiments. These results suggest that TRAF2 might be the adapter protein that recruits MLK3 to the TNFR. To further confirm that our results were not due to transfection artifact, we used mouse embryonic fibroblasts (MEFs) from wild-type and TRAF2-deficient mice to examine whether TRAF2 is required for TNF-α-induced MLK3 activation. The wild-type (TRAF2+/+) and TRAF2-deficient (TRAF2−/−) MEFs were treated with 10 nM TNF-α for 30 or 60 min and the endogenous MLK3 kinase activity was measured. Endogenous MLK3 was activated by about 4.5-fold after 30 or 60 min of stimulation by TNF-α in wild-type but not in TRAF2-deficient MEFs (Figure 2B, compare lanes 4 and 5 with 8 and 9). The time period for TNF-α treatment was chosen based on our results as shown in Figure 3. Taken together these results conclusively show that TRAF2 is necessary for TNF-α-mediated MLK3 activation.
TRAF2 is necessary for TNFα-induced MLK3 activation. (A) Overexpression of TRAF2 causes activation of MLK3. HEK-293 cells were transiently transfected with GST epitope-tagged MLK3 and Flag epitope-tagged various TRAF expression vectors, as indicated. The cells were starved for 12 h in 0.2% serum before harvesting 48 h after transfection. The recombinant MLK3 was pulled down by reduced GSH beads and subjected to kinase assay using bacterially expressed, SEK1 (K-R) protein as a specific substrate. The equal expression levels of MLK3 (second panel from top) and TRAF proteins (bottom panel) were detected by antibodies against epitopes, as indicated. The equal quantity of GST-SEK1 (K-R) substrate protein was judged by Coomassie stain. (B) TRAF2 is essential for MLK3 activation by TNF-α. The MEFs from wild-type (lanes 2-5) and TRAF2-deficient (lanes 6-9) mice were grown in serum-containing complete media and then starved for 12 h in 0.2% serum-containing media before TNF-α (10 nM) treatment for 30 or 60 min. The endogenous MLK3 was immunoprecipitated from these cells using MLK3-specific antibody and the immunoprecipitates were subjected to kinase assay as detailed above.
TNF-α induces the endogenous association of MLK3 with TRAF2. (A) MLK3 and TRAF2 associate endogenously in response to TNF-α in Jurkat cells. The Jurkat T cells were starved for 12 h in 0.2% serum-containing media and treated with 10 nM of TNF-α for indicated periods of time. The endogenous TRAF2 protein was immunoprecipitated and immune complex protein samples were blotted with anti-MLK3 antibody. (B) The endogenous interaction between MLK3 and TRAF2 is specific. The MEFs from wild-type and TRAF2-deficient mice were grown and treated with TNF-α as described above and the endogenous TRAF2 was immunoprecipitated, and any associated MLK3 protein was detected by anti-MLK3 antibody.
Association between endogenous MLK3 and TRAF2 is regulated by TNF-α
Given that TRAF2 protein can associate (Figure 1) with and activate ectopically expressed MLK3 (Figure 2A), we planned to test the association between TRAF2 and MLK3 under a more physiological condition. The Jurkat T cells were chosen for these experiments, since these cells respond very well to TNF-α treatment and express considerable amounts of endogenous MLK3. To examine the endogenous interaction between MLK3 and TRAF2, we starved Jurkat T cells in low serum (0.2% fetal bovine serum, FBS)-containing medium for 12 h and then treated with human TNF-α for different periods of time. The endogenous TRAF2 was immunoprecipitated and any associated MLK3 in the immune complex was blotted with antibody against MLK3. Endogenous MLK3 co-immunoprecipitated with TRAF2 in a time-dependent manner and the interaction was maximal at 30 min of TNF-α treatment and was undetectable by 120 min (Figure 3A). To further confirm that the endogenous interaction between MLK3-TRAF2 was not due to any artifacts of nonspecific interaction, we treated MEFs from wild-type and TRAF2-deficient mice with murine TNF-α, similar to Jurkat T cells. Endogenous TRAF2 was immunoprecipitated and blotted for any associated MLK3, similar to Jurkat T cells. The association between endogenous TRAF2 and MLK3 was only observed in TRAF2 immunoprecipitates from wild-type MEFs, but not in TRAF2-deficient MEFs, in response to TNF-α treatment (Figure 3B). This association between MLK3 and TRAF2 was also higher in wild-type MEFs at 30 min of TNF-α treatment (Figure 3B), as observed in Jurkat T cells. Therefore, it can be concluded that the interaction between MLK3 and TRAF2 is physiological and is regulated by TNF-α.
Mapping of in vivo interaction domains between MLK3 and TRAF2
The sequence analysis of TRAF family proteins reveals several conserved domains, such as RING, zinc-finger and TRAF domains, which are reported to mediate protein-protein interactions. To identify the domain(s) of TRAF2 that interacts with MLK3, we used several TRAF2 deletion constructs, as shown in Figure 4A. These TRAF2 mutant constructs were transfected into HEK-293 cells along with an MLK3 expression vector, as indicated in Figure 4B. First, the expression levels of all TRAF2 mutants, including the full-length proteins, were analyzed, and then the cell lysates with equal expression of TRAF2 mutant proteins were immunoprecipitated using anti-FLAG antibody. The association between MLK3 and TRAF2 mutant proteins was detected using an antibody against the GST tag present on recombinant MLK3. As per our expectation, MLK3 interacted with full-length and other TRAF2 mutant proteins, except for the TRAF2 mutant protein where the entire TRAF domain was deleted (Figure 4B, lane 4). Interestingly, the interaction between the entire TRAF domain and MLK3 was the least (Figure 4B, lane 5) whereas it was the highest with the N terminus of the TRAF domain (Figure 4B, lane 7). Our result is consistent with that of another protein, where it has been reported that the isolated TRAF domain does not interact with ASK1, despite that deletion of the TRAF domain from TRAF2 abolishes the interaction with full-length ASK1 19. Nevertheless, our results clearly suggest that TRAF2 interacts with MLK3 through the TRAF-N domain (Figure 4B).
Mapping the interaction domains of TRAF2 and MLK3. (A) TRAF2 mutants were used to determine the interaction domain(s). The domain structure of full-length TRAF2 is shown, with amino-acid number. The various TRAF2 mutants used are presented diagrammatically. (B) TRAF2 interacts with MLK3 through TRAF domain. HEK-293 cells were transfected with GST-tagged MLK3 and indicated Flag-tagged TRAF full-length or truncated mutant constructs. The TRAF proteins were immunoprecipitated by anti-FLAG antibody and any associated MLK3 was detected by anti-GST antibody (upper panel). The expression levels of MLK3 (middle panel) and TRAF mutant (lower panel) proteins were detected by antibodies against the tag epitopes. (C) MLK3 mutants used to determine the interaction domains(s). The domain structure of full-length MLK3 is shown; with amino-acid number. The various MLK3 are presented diagrammatically. (D) The C-terminal domain of MLK3 interacts with TRAF2. HEK-293 cells were transfected with full-length Flag-tagged TRAF2 and GST-tagged MLK3 deletion mutant or full-length expression vectors, as indicated. The MLK3 proteins were pulled down with GSH beads and any associated TRAF2 protein was detected with anti-FLAG antibody.
Similar to TRAF2, MLK3 also contains several conserved domains, which have been shown to mediate protein-protein interactions 24. Several deletion mutants (Figure 4C) of MLK3 protein were created to map the specific site on MLK3 required for interaction with TRAF2. These constructs were transfected into HEK-293 cells along with Flag-tagged, full-length TRAF2 expression vector. The interactions between TRAF2 and MLK3 mutant proteins were determined by immunoprecipitating TRAF2 by anti-FLAG antibody and blotting with anti-GST antibody for associated MLK3 mutant proteins. As per our earlier observation (Figure 1), full-length MLK3 interacted with TRAF2 whereas all other mutant proteins, except for the C-terminal domain of MLK3, failed to interact with full-length TRAF2 (Figure 4D, lane 10). These results suggest that MLK3 interacts with TRAF2 through its C-terminal regulatory domain.
TRAF2-MLK3 association is necessary for TNF-α-induced MLK3 activation
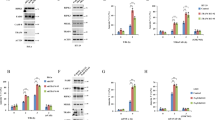
TRAF2 is an adapter protein and lacks any intrinsic kinase activity and thus we wanted to know how TRAF2 activates MLK3 kinase activity. One potential way by which TRAF2 might activate MLK3 is through protein-protein interaction in response to TNF-α treatment. To test whether direct interaction of TRAF2 with MLK3 activates MLK3 kinase activity in response to TNF-α, we treated Jurkat T cells with TNF-α for different time points, as indicated in Figure 5A. First, we optimized the conditions to immunoprecipitate equal amounts of total MLK3 and TRAF2-associated MLK3 by using MLK3 and TRAF2 antibodies, respectively (Figure 5A). Once we optimized how much of cell lysates were needed to immunoprecipitate equal amounts of MLK3 by both the antibodies, we immunoprecipitated comparable amounts of total and TRAF2-associated MLK3 as shown in Figure 5A, and measured MLK3 kinase activity by using SEK1 (K-R) protein as the substrate. As expected, our results showed about 4-fold activation of TRAF2-associated MLK3 when compared to equal amount of total MLK3 after 30 min of TNF-α treatment (Figure 5A, compare lane 5 with 6). These results clearly suggest that TNF-α-induced TRAF2-MLK3 interaction mediates MLK3 activation. To further confirm that the interaction between TRAF2 and MLK3 indeed is required for induction of MLK3 kinase activity, we attempted to compete off this interaction by using the TRAF-N domain that we had determined to be the minimal domain on TRAF2 for MLK3 interaction (Figure 4B). Constant amount of MLK3 and increasing quantity of cDNA expressing the TRAF-N domain (AA, 272-358) were transfected into HEK-293 cells, which were treated with either 10 nM TNF-α for 30 min, or left alone, as controls (Figure 5B). Equal amount of MLK3 was immunoprecipitated from these cells and subjected to kinase assay. Confirming our endogenous interaction data (Figure 5A), the MLK3 kinase activity was gradually attenuated by increasing doses of TRAF-N domain in TNF-α-treated cells but not in untreated cells (Figure 5B). These results clearly suggest that TNF-α induces the interaction between TRAF2 and MLK3 and this interaction leads to MLK3 activation.
TNF-α induces an association between TRAF2 and MLK3, and activates MLK3 kinase activity. (A) Association of TRAF2 with MLK3 increases its kinase activity. Jurkat T cells were starved for 12 h as indicated in Figure 3 and were treated with 10 nM TNF-α for indicated periods of time. Cells were lysed and comparable amounts of endogenous total MLK3 and MLK3 associated with TRAF2 were individually immunoprecipitated, and MLK3 kinase activities were measured using SEK1 (K-R) as substrate. (B) Interaction between TRAF2 and MLK3 is necessary for TNF-α-induced MLK3 kinase activation. HEK-293 cells were transfected with MLK3 and increasing concentrations of TRAF2 mutant constructs (i.e., TRAF2 AA 272-358) to compete off the binding between full-length endogenous TRAF2 and recombined MLK3. Some of these cells were treated with TNF-α as indicated for 30 min. The recombined MLK3 was immunoprecipitated and kinase activity was detected as described in Figure 2.
TRAF2 and MLK3 are necessary for TNF-α-induced JNK activation
Earlier we have shown that MLK3 was necessary for TNF-α-induced JNK activation 5 and MLK3 specifically mediated TNF-α-induced activation of JNK but not other MAPKs 5. To examine whether TRAF2 mediates TNF-α action on JNK through MLK3, we took advantage of TRAF2-deficient MEFs. These cells were transfected with the TRAF2 expression plasmid or used without transfection as controls. At 2 days after transfection, these cells were treated with TNF-α for 30 or 60 min and JNK activity was measured using bacterially expressed GST-c-JUN as the substrate. The basal activity of JNK in these cells was very low upon TNF-α treatment in the absence of endogenous TRAF2 (Figure 6A, lane 2) and was slightly elevated on ectopic expression of TRAF2, without TNF-α treatment (lane 3). However, JNK activity was increased by approximately 3.5-fold in these cells, when TRAF2 was ectopically expressed and the cells were treated with TNF-α for 30 or 60 min (Figure 6A). The MLK3 kinase activity was also estimated from these cells, which showed an activation profile similar to that of JNK (Figure 6B). On the basis of these results and our previously published reports 5, we conclude that TRAF2 is necessary for MLK3-mediated JNK activation by TNF-α.
TRAF2 and MLK3 are necessary for TNF-α-induced JNK activation. (A) JNK is the downstream target of MLK3, specifically induced by TNF-α-TRAF2 pathway. The TRAF2-deficient MEFs (i.e., TRAF2−/−) were treated with 10 nM TNF-α for 30 min (lane 2) or reconstituted with full-length TRAF2 expression vectors and then treated with TNF-α for 30 or 60 min (lanes 4 and 5). The endogenous JNK was immunoprecipitated and the kinase activity was measured using GST-Jun as the substrate. (B) Cell extracts as prepared in (A) were used to immunoprecipitate endogenous MLK3 and kinase activity was estimated as described in Figure 2. (C) MLK3 is necessary for TNF-α-induced JNK activation. The endogenous MLK3 in normal MEF cells was knocked down by transducing lentivirus containing MLK3-specific shRNA, as described in Materials and Methods. As controls, some cells were transduced with LacZ shRNA or left alone. TNF-α treatment was performed as in (A); and total cell lysates were blotted with indicated antibodies.
To further confirm that MLK3 is required for TNF-α-induced JNK activation through TRAF2, we used normal MEFs (i.e., TRAF2+/+) to knock down the endogenous MLK3 by transducing these cells with lentivirus-expressing MLK3-specific shRNA. Cells transduced with LacZ shRNA were used as controls (Figure 6C). These cells were treated with murine TNF-α for 30 min or left untreated as controls. Western blot analysis with anti-MLK3 antibody clearly showed about 95% knockdown of endogenous MLK3 by MLK3-specific shRNA (Figure 6C, lower panel, lanes 3 and 4) but not by LacZ shRNA (lanes 5 and 6). Equal amount of cell lysates was then blotted with phospho-JNK antibody to determine the activation status of endogenous JNK in these cells. Our results showed that TNF-α indeed activated endogenous JNK in control cells with endogenous MLK3 (Figure 6C, upper panel, lanes 2 and 6), however the activation of JNK by TNF-α was attenuated significantly when MLK3 was knocked down by MLK3-specific shRNA (lane 4). Collectively, these results convincingly show that the TRAF2-MLK3 pathway mediates the TNF-α-induced JNK activation.
Discussion
We have shown earlier that MLK3 was activated by TNF-α 5. In that study, we also reported that TNF-α-induced MLK3 activation specifically activated JNK but not p38 or ERK MAPKs 5, suggesting that TNF-α-induced MLK3 activity was actually specific for JNK activation. Here, we provide a mechanism by which TNF-α activates MLK3 and its downstream target JNK.
The family members of TRAF adapter proteins have been implicated in cytokine-mediated downstream signaling, where TRAF2, TRAF5 and TRAF6 have been shown to activate the JNK pathway 17. Our initial expectation was that MLK3 might interact with all three TRAF proteins, which are already implicated in the activation of JNK. Our results clearly suggest that all three TRAF proteins (i.e., TRAF2, TRAF5 and TRAF6) can interact with ectopically expressed MLK3 (Figure 1), but only TRAF2 can activate MLK3 kinase activity (Figure 2A). It remains a possibility that under specific physiological conditions, and in response to specific stimuli, TRAF5 and TRAF6 proteins might also trigger MLK3 activation, which is yet to be determined. The interaction between TRAF2 and MLK3 is physiological based on the fact that endogenous TRAF2 can interact with MLK3 in response to the ligand of MLK3, i.e., TNF-α (Figure 3), in a time-dependent manner.
TRAF2 interaction with other MAP3Ks has been previously shown to cause activation 18, 19, 20, 21, but how the interaction per se leads to increase in the catalytic activities of these kinases is still not known. We also observed that the interaction between MLK3 and TRAF2 was essential for TNF-α-induced activation of MLK3 because the TRAF2-associated MLK3 was 4.5-fold more active compared to the total cellular pool of MLK3 (Figure 5A). Furthermore, we also confirmed that the activation of MLK3 by TNF-α was through TRAF2 interaction because MLK3 activation was attenuated in a dose-dependent manner by the TRAF-N protein (Figure 5B) that interacts with MLK3 (Figure 4A). Thus collectively, we can conclude that the TRAF2-MLK3 interaction is essential for MLK3 activation by TNF-α. During the preparation of this paper, similar findings were reported by Korchnak et al. 25. We reported earlier that in addition to TNF-α, the bioactive membrane lipid ceramides also activate MLK3 5 and therefore one potential mechanism by which TRAF2 interaction with MLK3 can lead to its activation might be by recruiting MLK3 to the membrane. When MLK3 is recruited to the membrane, the ceramides generated by TNF-α-induced sphingomyelinase activation 26 might modulate MLK3 kinase activation, which is yet to be determined. We also examined a potential association between MLK3 and TRAF2 induced by ceramide in Jurkat cells. The ceramide concentration that activated the endogenous MLK3 in Jurkat cells did not induce any interaction between TRAF2 and MLK3 (data not shown), suggesting that mechanisms of MLK3 activation induced by TNF-α and ceramides are distinct.
TRAF2 has been shown to cause activation of both JNK and NF-κB pathways 17, although now it is known that TRAF2 primarily activates the JNK pathway rather than NF-κB, as MEFs from TRAF2-deficient mice were defective in JNK activation but not in NF-κB pathway activation 27. One report also implicated MLK3 in the activation of NF-κB pathway in T cells through activating IKKs 28; however we were unable to reproduce similar effect of MLK3 on NF-κB activation in T cells. Interestingly other MAP3K members, such as ASK1 and MEKK1, are also activated through TNF-α-induced TRAF2 interaction and have been reported to activate downstream JNK pathway 18, 19, 21, 29. However, studies in mice deficient for these kinases could not support an essential role of these kinases in TNF-α-induced JNK activation, suggesting some other MAP3K members might be essential for TNF-α-induced JNK activation 30, 31. Thus, we suggest that MLK3 is a primary MAP3K in the TNF-JNK pathway, which is also supported by an earlier study of MLK3 knockout MEFs 32. In addition, we also observed that MLK3-induced JNK activation does require TRAF2 (Figure 6A).
On the basis of our current data and several published reports, we propose a model for JNK and NF-κB pathway activation through the TNFRs-TRAF2 pathway (Figure 7). The TNF-α-induced JNK and NF-κB pathways bifurcate at the level of TRAF2, where MLK3 interacts with TRAF2 on TNF-α stimulation and phosphorylates MKK4 and MKK7 (two MAPKK members in the JNK pathway), which then phosphorylate and activate downstream JNK that finally translocates to the nucleus and regulates AP1-mediated transcription. Because TRAF2 deficiency purely downregulates JNK and not NF-κB pathway, TNF-α might use other TRAFs, such as TRAF5, to activate NIK, which eventually leads to NF-κB activation. In addition, the death domain-containing protein RIP is required for TNF-α-induced NF-κB activation. Because we did not observe any NF-κB activation in Jurkat T cells on MLK3 overexpression (data not shown), we can conclude that TNF-α-induced interaction between MLK3 and TRAF2 primarily promotes activation of the JNK pathway and not NF-κB.
A model of MLK3 activation by TRAF2 through TNF pathway. The proinflammatory cytokine TNF-α binds to its receptor, TNFR, that allows death domain-containing adaptor protein TRADD to associate with the death domain (DD) of TNFR at the cytoplasmic end. The TRAF2 binds with TRADD and MLK3. This protein-protein interaction allows MLK3 activation and its downstream target JNK through MKK4 and MKK7 (two MAPKK). The other details about NF-κB activation by TRAF2 are described in the text.
In conclusion, our data provide an insight into the mechanism by which TNF-α activates MLK3. Our biochemical data clearly show that the TRAF2-MLK3 interaction is necessary for MLK3 and its downstream target JNK activation. The interaction between MLK3 and TRAF2 is physiological because these proteins interact endogenously on TNF-α treatment in a time-dependent manner and the activation of MLK3 was specifically blocked by the mutant form of TRAF2 that binds to the MLK3 protein. We are currently in the process of determining in details, how the interaction between TRAF2 and MLK3 leads to MLK3 activation.
Materials and Methods
Cell culture and treatments
Human embryonic kidney 293 cells, TRAF2-deficient (TRAF2−/−) and normal MEFs were maintained in Dulbecco's modified Eagle's medium containing 10% FBS. Jurkat T cells were maintained in RPMI medium containing 4.5 g/l glucose, 10 mM HEPES, 1.0 mM sodium pyruvate, 10% FBS and 50 μM β-ME. For TNF-α treatment, either murine TNF-α (Roche Applied Science, Mannheim, Germany) for cell lines of murine origin, or human TNF-α (Roche Applied Science) for cell lines of human origin, were used, as indicated. The cells were starved in 0.2% serum-containing media for 12 h before treatment with 10 nM TNF-α for the indicated periods of time.
Plasmids, transfection and MLK3 knockdown
The Flag-tagged TRAF2 clones were obtained from Dr James R Woodgett (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada) as described 18. Myc-tagged TRAF1 and TRAF3 were obtained from Dr Bharat Agarwal (MD Anderson Cancer Center, Houston, TX, USA) and all other TRAFs (i.e., TRAF5 and 6) were from Dr Woodgett's laboratory 18. The MLK3 clones were constructed in our laboratory, as described earlier 24, 33. For cDNA transfection, in most cases, 1 μg of various cDNAs was taken, otherwise as indicated. The various cell types used were transfected with the appropriate expression vectors using LipofectAMINE (Invitrogen, Carlsbad, CA, USA) reagent as per the manufacturer's instructions. The endogenous MLK3 was knocked down using MLK-specific shRNA. The shRNA oligos were synthesized commercially (Invitrogen):
Upper strand, CAC CGG CTG GAA ACG CGA GAT CCC GAA GGA TCT CGC GTT TCC AGC C;
Lower strand: AAA AGG CTG GAA ACG CGA GAT CCT TCG GGA TCT CGC GTT TCC AGC C
The upper and lower strands were annealed and subcloned into entry vector pENTR/H1/TO vector (Invitrogen) and recombinant lentivirus was produced using Virapower T-Rex Lentiviral expression system (Invitrogen) following manufacture's instruction. Twenty MOIs were used to knock down the endogenous MLK3 in MEFs.
Immunoblot analysis
For immunoblotting, equal protein content of cell extracts or the immunoprecipitated protein samples were taken. The proteins were separated on denaturing SDS-PAGE and transferred onto polyvinylidene difluoride membrane and blotted with antibodies as indicated. Antibodies used were anti-FLAG (Sigma, St Louis, MO, USA); anti-GST (Millipore, Billerica, MA, USA); anti-TRAF2 (Cell Signaling, Danvers, MA, USA); anti-JNK (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-phospho-JNK (Promega, Madison, WI, USA). The antibody for immunoprecipitating endogenous JNK was provided by Dr Joseph Avruch (Massachusetts General Hospital, Boston, MA, USA). For immunoprecipitating endogenous MLK3, the antibody against the C-terminal peptide of MLK3 was developed in our laboratory, as described earlier 24, 33. For blotting endogenous MLK3, the antibody against the N-terminal peptide of MLK3 developed in our laboratory was used.
Immunoprecipitation and kinase assay
Jurkat T cells, TRAF2+/+, TRAF2−/− MEFs and HEK-293 cells were lysed in lysis buffer as described previously 2. The cell extracts were clarified by centrifugation at 15 000 × g for 5 min, and protein contents were estimated using the Bradford method. Immunoprecipitation and GST pull-down assays were performed by either specific antibodies or GSH beads. After thorough washing, the immunoprecipitates were either processed for immunoblotting or used for kinase assay as described previously 2, 5.
References
Gallo KA, Johnson GL . Signalling: mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3:663–672.
Rana A, Gallo K, Godowski P, et al. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem 1996; 271:19025–19028.
Hirai S, Katoh M, Terada M, et al. MST/MLK2, a member of the mixed lineage kinase family, directly phosphorylates and activates SEK1, an activator of c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem 1997; 272:15167–15173.
Fan G, Merritt SE, Kortenjann M, et al. Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. J Biol Chem 1996; 271:24788–24793.
Sathyanarayana P, Barthwal MK, Kundu CN, et al. Activation of the Drosophila MLK by ceramide reveals TNF-α and ceramide as agonists of mammalian MLK3. Mol Cell 2002; 10:1527–1533.
Naismith JH, Sprang SR . Modularity in the TNF-receptor family. Trends Biochem Sci 1998; 23:74–79.
Banner DW, D'Arcy A, Janes W, et al. Crystal structure of the soluble human 55 kD TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 1993; 73:431–445.
Chan FK, Chun HJ, Zheng L, et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000; 288:2351–2354.
Wajant H, Pfizenmaier K, Scheurich P . Tumor necrosis factor signaling. Cell Death Differ 2003; 10:45–65.
Chung JY, Park YC, Ye H, et al. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 2002; 115:679–688.
Rothe M, Pan MG, Henzel WJ, et al. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995; 83:1243–1252.
Arch RH, Gedrich RW, Thompson CB . Tumor necrosis factor receptor-associated factors (TRAFs) – a family of adapter proteins that regulates life and death. Genes Dev 1998; 12:2821–2830.
Cao Z, Xiong J, Takeuchi M, et al. TRAF6 is a signal transducer for interleukin-1. Nature 1996; 383:443–446.
Croston GE, Cao Z, Goeddel DV . NF-κB activation by interleukin-1 (IL-1) requires an IL-1 receptor-associated protein kinase activity. J Biol Chem 1995; 270:16514–16517.
Cao Z, Henzel WJ, Gao X . IRAK: a kinase associated with the interleukin-1 receptor. Science 1996; 271:1128–1131.
Xing L, Venegas AM, Chen A, et al. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev 2001; 15:241–253.
Song HY, Regnier CH, Kirschning CJ, et al. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA 1997; 94:9792–9796.
Hoeflich KP, Yeh WC, Yao Z, et al. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1). Oncogene 1999; 18:5814–5820.
Nishitoh H, Saitoh M, Mochida Y, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 1998; 2:389–395.
Takaesu G, Kishida S, Hiyama A, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 2000; 5:649–658.
Baud V, Liu ZG, Bennett B, et al. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev 1999; 13:1297–1308.
Malinin NL, Boldin MP, Kovalenko AV, et al. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 1997; 385:540–544.
Yuasa T, Ohno S, Kehrl JH, Kyriakis JM . Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem 1998; 273:22681–22692.
Mishra R, Barthwal MK, Sondarva G, et al. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem 2007; 282:30393–30405.
Korchnak AC, Zhan Y, Aguilar MT, et al. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell Signal 2009; 21:1620–1625.
Kronke M . Involvement of sphingomyelinases in TNF signaling pathways. Chem Phys Lipids 1999; 102:157–166.
Yeh WC, Shahinian A, Speiser D, et al. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 1997; 7:715–725.
Hehner SP, Hofmann TG, Ushmorov A, et al. Mixed-lineage kinase 3 delivers CD3/CD28-derived signals into the IκB kinase complex. Mol Cell Biol 2000; 20:2556–2568.
Baud V, Karin M . Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 2001; 11:372–377.
Yujiri T, Ware M, Widmann C, et al. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc Natl Acad Sci USA 2000; 97:7272–7277.
Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001; 2:222–228.
Brancho D, Ventura JJ, Jaeschke A, et al. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol 2005; 25:3670–3681.
Barthwal MK, Sathyanarayana P, Kundu CN, et al. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem 2003; 278:3897–3902.
Acknowledgements
We thank Dr James R Woodgett (Mount Sinai Hospital, Toronto, Canada) for providing the TRAF constructs and TRAF2 knockout MEFs. This work was supported by National Institutes of Health (NIH) Grant GM55835 and Veterans Affairs Merit Award (to AR). BR was supported by NIH (CA121221) and Veterans Affairs Merit Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sondarva, G., Kundu, C., Mehrotra, S. et al. TRAF2-MLK3 interaction is essential for TNF-α-induced MLK3 activation. Cell Res 20, 89–98 (2010). https://doi.org/10.1038/cr.2009.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2009.125
Keywords
This article is cited by
-
cIAP1/TRAF2 interplay promotes tumor growth through the activation of STAT3
Oncogene (2023)
-
Identification of PTPN1 as a novel negative regulator of the JNK MAPK pathway using a synthetic screening for pathway-specific phosphatases
Scientific Reports (2017)
-
TRAF2 is a biologically important necroptosis suppressor
Cell Death & Differentiation (2015)
-
LRRC19 expressed in the kidney induces TRAF2/6-mediated signals to prevent infection by uropathogenic bacteria
Nature Communications (2014)