Abstract
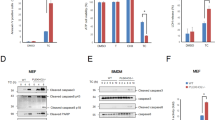
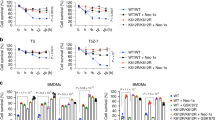
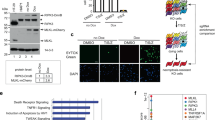
Tumor necrosis factor α (TNF-α) is a pro-inflammatory cytokine capable of inducing extrinsic apoptosis and necroptosis. Tumor necrosis factor receptor-associated factor 6 (TRAF6), an E3 ligase, is a member of the TRAF family of proteins, which mediates inflammatory signals by activating nuclear factor kappa B (NFкB) and mitogen-activated protein kinase (MAPK). Although the functions of TRAF6 have been identified, its role in TNF-α-induced cell death remains poorly understood. Here, we report that TRAF6 is a negative modulator of TNF-α-induced cell death but does not affect TNF-α-induced NFκB activation. TRAF6 deficiency accelerates both TNF-α-induced apoptosis and necroptosis; however, the acceleration can be reversed by reconstituting TRAF6 or TRAF6C70A, suggesting that E3 ligase activity is not required for this activity. Mechanistically, TRAF6 directly interacts with RIPK1 during TNF-α-induced cell death signaling, which prevents RIPK1 from interacting with components of the cell death complex such as itself, FADD or RIPK3. These processes suppress the assembly of the death complex. Notably, IKK was required for TRAF6 to interact with RIPK1. In vivo, Traf6-/- embryos exhibited higher levels of cell death in the liver but could be rescued by the simultaneous knockout of Tnf. Finally, TRAF6 knockdown xenografts were highly sensitive to necroptotic stimuli. We concluded that TRAF6 suppresses TNF-α-induced cell death in coordination with IKK complexes in vivo and in vitro by suppressing the assembly of cell death complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72.
Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott M. Tumour necrosis factor signalling in health and disease. F1000Res. 2019;8:111.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64.
Kumari S, Van TM, Preukschat D, Schuenke H, Basic M, Bleich A, et al. NF-kappaB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation. Life Sci Alliance. 2021;4:e202000956.
Seo J, Nam YW, Kim S, Oh DB, Song J. Necroptosis molecular mechanisms: recent findings regarding novel necroptosis regulators. Exp Mol Med. 2021;53:1007–17.
Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62.
Park HH. Structure of TRAF family: current understanding of receptor recognition. Front Immunol. 2018;9:1999.
Yamamoto M, Gohda J, Akiyama T, Inoue JI. TNF receptor-associated factor 6 (TRAF6) plays crucial roles in multiple biological systems through polyubiquitination-mediated NF-kappa B activation. P Jpn Acad B-Phys. 2021;97:145–60.
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24.
Schimmack G, Schorpp K, Kutzner K, Gehring T, Brenke JK, Hadian K, et al. YOD1/TRAF6 association balances p62-dependent IL-1 signaling to NF-kappaB. Elife. 2017;6:e22416.
Fang J, Bolanos LC, Choi K, Liu X, Christie S, Akunuru S, et al. Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia. Nat Immunol. 2017;18:236–45.
Chen X, Chen Y. Ubiquitination of cGAS by TRAF6 regulates anti-DNA viral innate immune responses. Biochem Biophys Res Commun. 2019;514:659–64.
Li T, Qin JJ, Yang X, Ji YX, Guo F, Cheng WL, et al. The ubiquitin E3 ligase TRAF6 exacerbates ischemic stroke by ubiquitinating and activating Rac1. J Neurosci. 2017;37:12123–40.
Sun H, Li XB, Meng Y, Fan L, Li M, Fang J. TRAF6 upregulates expression of HIF-1alpha and promotes tumor angiogenesis. Cancer Res. 2013;73:4950–9.
Zhang X, Li CF, Zhang L, Wu CY, Han L, Jin G, et al. TRAF6 restricts p53 mitochondrial translocation, apoptosis, and tumor suppression. Mol Cell. 2016;64:803–14.
Li JA, Kuang TT, Pu N, Fang Y, Han X, Zhang L, et al. TRAF6 regulates YAP signaling by promoting the ubiquitination and degradation of MST1 in pancreatic cancer. Clin Exp Med. 2019;19:211–8.
Zhou C, Lu C, Pu H, Li D, Zhang L. TRAF6 promotes IL-4-induced M2 macrophage activation by stabilizing STAT6. Mol Immunol. 2020;127:223–9.
Meng Y, Liu C, Shen L, Zhou M, Liu W, Kowolik C, et al. TRAF6 mediates human DNA2 polyubiquitination and nuclear localization to maintain nuclear genome integrity. Nucleic Acids Res. 2019;47:7564–79.
Mishra AK, Sachan N, Mutsuddi M, Mukherjee A. TRAF6 is a novel regulator of Notch signaling in Drosophila melanogaster. Cell Signal. 2014;26:3016–26.
Wang Z, Liu Y, Huang S, Fang M. TRAF6 interacts with and ubiquitinates PIK3CA to enhance PI3K activation. FEBS Lett. 2018;592:1882–92.
Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3:ra42.
Ling T, Weng GX, Li J, Li C, Wang W, Cao L, et al. TARBP2 inhibits IRF7 activation by suppressing TRAF6-mediated K63-linked ubiquitination of IRF7. Mol Immunol. 2019;109:116–25.
Hindi SM, Paul PK, Dahiya S, Mishra V, Bhatnagar S, Kuang S, et al. Reciprocal Interaction between TRAF6 and notch signaling regulates adult myofiber regeneration upon injury. Mol Cell Biol. 2012;32:4833–45.
Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife. 2013;2:e00785.
Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–96.
Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–8.
Dainichi T, Matsumoto R, Mostafa A, Kabashima K. Immune Control by TRAF6-Mediated Pathways of Epithelial Cells in the EIME (Epithelial Immune Microenvironment). Front Immunol. 2019;10:1107.
Li JD, Liu NA, Tang L, Yan B, Chen X, Zhang JL, et al. The relationship between TRAF6 and tumors. Cancer Cell Int. 2020;20:429.
Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191:1395–411.
Vlantis K, Polykratis A, Welz PS, van Loo G, Pasparakis M, Wullaert A. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut. 2016;65:935–43.
Kisiswa L, Fernandez-Suarez D, Sergaki MC, Ibanez CF. RIP2 gates TRAF6 interaction with death receptor p75(NTR) to regulate cerebellar granule neuron survival. Cell Rep. 2018;24:1013–24.
He L, Wu X, Siegel R, Lipsky PE. TRAF6 regulates cell fate decisions by inducing caspase 8-dependent apoptosis and the activation of NF-kappaB. J Biol Chem. 2006;281:11235–49.
Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Curr Biol. 2006;16:1666–71.
Fan Z, Wu Z, Yang B. The Effect of miR-361-3p targeting TRAF6 on apoptosis of multiple myeloma cells. J Microbiol Biotechnol. 2021;31:197–206.
Meng Q, Liang C, Hua J, Zhang B, Liu J, Zhang Y, et al. A miR-146a-5p/TRAF6/NF-kB p65 axis regulates pancreatic cancer chemoresistance: functional validation and clinical significance. Theranostics. 2020;10:3967–79.
Xie C, Zhang LZ, Chen ZL, Zhong WJ, Fang JH, Zhu Y, et al. A hMTR4-PDIA3P1-miR-125/124-TRAF6 regulatory axis and its function in NF kappa B signaling and chemoresistance. Hepatology. 2020;71:1660–77.
Yoon K, Jung EJ, Lee SR, Kim J, Choi Y, Lee SY. TRAF6 deficiency promotes TNF-induced cell death through inactivation of GSK3 beta. Cell Death Differ. 2008;15:730–8.
Ichikawa D, Funakoshi-Tago M, Aizu-Yokota E, Sonoda Y, Inoue J, Kasahara T. TNF-receptor associated factor 6-deficient fibroblast is sensitive to the TNF-alpha-induced cell death: involvement of reactive oxygen species. Biochem Biophys Res Commun. 2006;351:93–8.
Strickson S, Emmerich CH, Goh ETH, Zhang J, Kelsall IR, Macartney T, et al. Roles of the TRAF6 and Pellino E3 ligases in MyD88 and RANKL signaling. Proc Natl Acad Sci USA. 2017;114:E3481–E9.
Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. Plos One. 2008;3:e4064.
Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X, et al. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat Commun. 2016;7:11267.
Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFκB and MAPK pathways in response to IL-1 and RANKL. Plos One. 2008;3:e4064.
Sabio G, Davis RJ, editors. TNF and MAP kinase signalling pathways. Seminars in immunology; 2014: Elsevier.
Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92–103.
Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37.
Dondelinger Y, Delanghe T, Priem D, Wynosky-Dolfi MA, Sorobetea D, Rojas-Rivera D, et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat Commun. 2019;10:1729.
Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Molecular cell. 2015;60:63–76.
Wang J, Wu X, Jiang M, Tai G. Mechanism by which TRAF6 participates in the immune regulation of autoimmune diseases and cancer. BioMed Research International. 2020;2020:4607197.
Seo J, Seong D, Nam YW, Hwang CH, Lee SR, Lee CS, et al. Beclin 1 functions as a negative modulator of MLKL oligomerisation by integrating into the necrosome complex. Cell Death Differ. 2020;27:3065–81.
Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8:339ra69.
Seong D, Jeong M, Seo J, Lee JY, Hwang CH, Shin HC, et al. Identification of MYC as an antinecroptotic protein that stifles RIPK1-RIPK3 complex formation. Proc Natl Acad Sci USA. 2020;117:19982–93.
Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275–88.
Townsend PA, Kozhevnikova MV, Cexus ONF, Zamyatnin AA Jr, Soond SM. BH3-mimetics: recent developments in cancer therapy. J Exp Clin Cancer Res. 2021;40:355.
Chie K, Katsuo N, Yoshiyuki T, Den-Ichi M, Gen-Ichiro S. Constitutive expression of TNF-α and-β genes in mouse embryo: roles of cytokines as regulator and effector on development. Int J Biochem. 1994;26:111–9.
Kamiya A, Gonzalez FJ. TNF-alpha regulates mouse fetal hepatic maturation induced by oncostatin M and extracellular matrices. Hepatology. 2004;40:527–36.
Tiegs G, Horst AK. TNF in the liver: targeting a central player in inflammation. Semin Immunopathol. 2022;44:445–59.
Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;156:997–1007.
Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9:e3465.
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (MSIP) (2015R1A3A2066581), in part by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1A5A1019023), and in part by the Brain Korea 21(BK21) FOUR program.
Author information
Authors and Affiliations
Contributions
C-SL designed, performed, and interpreted all experiments and prepared the manuscript. GH prepared materials and performed the xenograft model experiment. YWN and CHH designed and interpreted the experimental results. JS directed the experiments and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The mouse embryo and xenograft model studies were approved by the Yonsei University Institutional Animal Care and Use Committee (IACUC-A-202111-1368-01 and IACUC-A-202205-1465-01).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, CS., Hwang, G., Nam, Y.W. et al. IKK-mediated TRAF6 and RIPK1 interaction stifles cell death complex assembly leading to the suppression of TNF-α-induced cell death. Cell Death Differ 30, 1575–1584 (2023). https://doi.org/10.1038/s41418-023-01161-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-023-01161-w