Abstract
NO is now known to be an important messenger molecule in biology. It regulates a variety of functions within cells and tissues including vasodilation, neurotransmission and immunological process. This review will focus on the nitric oxide synthase gene family and recent progress on molecular genetic analysis of NOS1, NOS2 and NOS3 genes.
Similar content being viewed by others
Introduction
Nitric Oxide and Nobel Prize
Scientific breakthrough often comes from unexpected observations, and imaginative adventurous research. In the early 80's at the New York Laboratory of Suny Health Centre, Dr. Robert Furchgott was presented contradicting results by his two technicians. One technician always found the acetycholine relaxed the blood vessel, whereas the other found it always caused contraction. Furchgott noticed that one technician handled the vessels roughly, and inadvertently rubbed off the thin layer of endothelium from the surface of the vessel, whilst the other was careful, and kept the endothelium intact. He then realized that an intact endothelium was a prerequisite for the relaxant effect of acetycholine1. By separating the endothelium from the smooth muscle, he showed that a labile factor was released from the endothelium, he named it the “endothelium-dependent relaxing factor” (EDRF).
Since then the race to hunt down this mysterious factor has been fiercely contested. The three winners of this race have been awarded the Nobel Prize for physiology/medicine this year. They are pharmacologists Robert Furchgott of the State University of New York; Louis Ignarro of the University of California, Los Angeles2; and Ferid Murad of University of Texas Medical School in Houston3. Although many scientists feel that a fourth name should also be recognized-Salvador Moncada, current director of the Wolfson Institute for Biomedical Research at University College London for his contribution to the conclusion that EDRF and nitric oxide was identical4, 5.
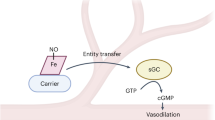
NO regulates vasodilation, neurotransmission and immunological process
In late 80's these pioneer scientists discovered that a gas, Nitric Oxide(NO), previously considered to be merely atmospheric pollutant, is a critical signaling molecule for endothelium-dependent relaxation which occurs in response to a wide variety of stimuli, including acetylcholine, bradykinin, substance P, thrombin, adenine nucleotide and calcium ionophore A23187. It increases in the blood flow through arteries, microvessels and some veins. It is now clear that the endothelium-dependent relaxation, described by Furchgott and the others, is just one of the myriad mediator functions of NO. NO also carries important information in the nervous system: In the brain, NO has been shown to be a neurotransmitter, and plays an important role in learning and memory6. In male, it is a message that translates sexual excitement into an erect penis. Pfizer's blockbuster drug Viagra reverses impotence by enhancing a NO-stimulated pathway. At a time when vascular pharmacologists were pursuing the identity of EDRF, immunologists were independently exploring the cytotoxic properties of macrophages that appeared to be dependent on the generation of large amounts of NO in response to infection7, 8. The immune system uses NO in fighting virus, bacteria, parasites, and tumors. By early 1990's, scientists in distinct disciplines-immunology, cardiovascular physiology and neuroscience-suddenly realized they were studying the same molecule, NO.
Nitric oxide gene family
NO is a highly labile gas, and a very small compound that is not stored, but diffuses from its site of formation to its site of action (since it is both water/lipid soluble, it diffuses freely within tissues). Because it contains an unpaired electron, it is extremely active. It binds to the heme moiety of guanylate cyclase, and causes a greater than 400-fold activation of the enzyme. NO is formed directly from the guanidino nitrogen of the L-arginine by nitric oxide synthase(NOS) through a process that consumes five electrons, and results in the formation of L-citrulline (Fig 1). NOS is an unusual oxidative enzyme, in that most other enzymes consume only one or two electrons for a similar function.
Biosynthesis of NO. L-arginine is converted to No in two successive steps ofwhich a two-electron oxidation of L-arginine to Nw-hydroxy-L-argninie is the first step, then converted to NO and citrulline, utilizing one and half NADPH and O2. Both steps require Ca2+ and calmodulin as activators and are accelerated by tetrahydrobiopterin.
The structures and functions of NO synthases have been clarified by molecular cloning of the cDNA from the brain (NOS1)9, endothelium (NOS3)10, macrophages and other type of inducible cells (NOS2)11 (Fig 2). Three forms of the NO synthase enzyme are known: NOS3 being initially isolated from the endothelium, NOS1 being isolated from brain cerebellum, and NOS2 being isolated from murine macrophages and human hepatocytes and chondrocytes. The molecular cloning, functional analysis and crystal structure study data revealed that NOS gene family shares similar compositions with each other. Comparison of the deduced sequence of the human inducible NO synthase (NOS2) with those of the two other human NO synthases shows 51% and 54 % identity and 68 % and 70 % similarity with the endothelial (NOS3) and neuronal (NOS1) NO synthase, respectively. These NOS enzymes all have two domains: N-terminal half of Heme-oxygenase domain with tetrahydrobiopterin, heme and arginine binding sites, and C-terminal half of P-450 reductase domain with the recognition sites for NADPH, as well as for flavin mononucleotide (FMN) and flavin adenosine dinucleotide(FAD). Cytochrome P-450 reductase is the only other mammalian enzyme known to contain these recognition sites (Fig 2).
Sequence homologies of molecular isoforms of NOS. All NOS enzymes have consensus binding sites for Arginine (Arg), Heme, FAD (flavin adenine dinucleotide), FMN (flavin mononucleotide), NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) and calmodulin (Cal). NOS1 has PDZ (Postsynaptic density protein, Disk large and Zo-1) binding domain. NOS3 has myristoylation (M) and Caveolin(C) binding sites although the site of Caveolin are only localized between amino acids 310 and 570. NOS2 cDNA, both NOS2 (murine) and NOS2 (human chondrocyte), is the shortest member of the family, lacking a 45-amino acid region (represented by the broken line) from the middle of their molecule. Cytochrome P450 reductase has transmembrane domain (TMD).
Both NOS3, and NOS1 enzymes are constitutively expressed in the sense that their activation does not require new enzyme protein synthesis. For instance, the NOS1 is activated by glutamate-induced increase in intracellular Ca2+ level which in turn activates NOS via calmodulin. NOS3 is also a constitutive enzyme, being similarly activated by the increase in intracellular Ca2+ level. Agonists such as acetylcholine and bradykinin activate muscarinic or bradykinin receptor on the endothelial cells to generate Ca2+ which then stimulates NOS through binding to calmadulin. Constitutive NOS enzymes account for the role of NO in mediating rapid events, such as neurotransmission, and blood vessel dilatation. In constrast, the inducible nitric oxide synthase, NOS2, which mediates the production of large amount of NO, can be activated by endotoxin and cytokines.
Recent progress in genetic study of NOS gene family
Soon after the first NOS has been cloned in 19919, we have mapped this gene on the human chromosome 12q12-q24 in 199212. In 1993, the fluorescence in situ hybridization has been used to pinpoint the gene on 12q24.313, followed by identifying the highly polymorphic markers for NOS1 gene14. This was succeeded by establishing the linkage map of the gene location, which leads to the identification of the human disease association with NOS1 gene. It turns out NOS1 is a susceptibility locus for infantile pyloric stenosis (PS), one of the most common and lethal condition in new born babies. In the 27 family studies, there were significant overall transmission disequilibrium between PS and NOSla (P < 0.006)15. Coincidentally, the most evident effect of disrupting the NOS1 gene in mice is the development of grossly enlarged stomach, with hypertrophy of the pyloric sphincter and the circular muscle layer16. This phenotype resembles the human disorder infantile pyloric stenosis, in which gastric outlet obstruction is associated with the lack of NADH neurons in the pylorus.
In order to hunt down the other human diseases in which NOS genes may be involved in, we and others also quickly carried out gene mapping studies, polymorphic marker scans, and disease association studies for the NOS2 and NOS3 genes. The NOS3 have been mapped to 7q35-q36 and NOS2 to 17q11.217. We have discovered the NOS gene family is a dispersed gene family18. Although NOS1 and NOS3 genes have been shown to be only a single chromosomal localization, we have found multiple copies of inducible nitric oxide synthase gene-like sequences in the human genome. In human chromosome 17, there are several positive in situ hybridization signals on both arms of chromosome, spanning from 17p11.2 to 17q11.217. So far, only NOS2A, which is located on 17q11.2, has been found to code for functional cDNAs. The other two NOS2-1ike sequences, NOS2B and NOS2C, have not been found to contain functional cDNAs (Tab 1)19. The genomic structures of the NOS1, NOS2 and NOS3 gene were also determined by our group and others (Tab 2)20.
As expected from physiological role of NOS3 gene, the NOS3 gene knock out mice has been shown to have phenotype of hypertension21. NOS2 knock out mice has been shown to be uniformly susceptible to infection by many pathogens, including tuberculosis22, 23. Therefore, it is one of the crucial genes to the host defence against many lethal pathogens. Recently, we have identified a highly polymorphic microsatellite marker in the human NOS2 promoter region24, and found a strong positive allele association between human NOS2 gene polymorphism and fetal cerebra malaria25. Infectious diseases are still the major health risks in developing countries. Future study of the NOS2 gene may help to unlock new methods to combat these diseases.
Alfred Nobel was prescribed nitroglycerin, one of the key components of dynamite, to ease his chest pain when he contracted heart disease. It took 100 years until it was clarified that nitroglycerin acts by releasing nitric oxide gas. However, it also opened floodgate for medical articles detailing the biological activity of nitric oxide (NO) which are now flooding the scientific journals at a pace of 500 papers per month. There are more than 22,000 medical articles that dealt with nitric oxide, yet nitric oxide was not even a subject 12 years ago. It is impossible for any review to cover such a vast amount of information now. The only hope we have is that this rapid progress of Nitric Oxide Research will bring more benefit for human health.
References
Furchgott RF, Zawadzki JV . The obligatory role of endothelial clues in the relaxation arterial smooth muscle by acetylcholine. Nature 1980; 288:373–6.
Ignarro IJ . Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Phar- macol Toxicol 1990; 30:535–60.
Rapoport RM, Draznin MB, Murad F . Endothelium-dependent relaxation in rat aorta may be maediated through cyclic GMP-dependent protein phosphorylation. Nature 1983; 306:174–6.
Palmer RM, Ferrige AG, Moncada S . Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 337:524–6.
Howlett R . Nobel award stirs up debate on nitric oxide breakthrough. Nature 1998; 395:625–6.
Garthwaite J . Garthwaite, nitric oxide and cell-cell signaling in the nervous system. Trends Neurosci 1991; 14:60–7.
Marlett MA . Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci 1989; 14:488–92.
Nathan CF, Hibbs JB Jr . Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 1991; 3:65–70.
Bredt DS, Hwang PH, Lowenstein C, Cloned and expressed nitric oxide synthase structurally resemble cytochromeP-450 reductase. Nature 1991; 351:714–8.
Busconi L, Michel T . Endothelial nitric oxide synthase. N-terminal myristolation determines subcellular localzation. J Bio Chem 1993; 268:8410–3.
Charles IG, Palmer MJ, Hickery MS, Baylisa MT, Chubb AP, Hall VS, Moss DW, Moncada S . Cloning, characterization and expression of cDNA encoding an inducible NO synthase from the human chondrocyte Proc Natl Acad Sci USA 1993; 90:11419–23.
Kishimoto J, Spurr N, Liao M, Liu L, Emson PC, Xu W . Localization of brain nitric oxide synthase (NOS) to human chromosome12. Genomics 1992; 14:802–4.
Xu W, Gorman P, Sheer D, Bates G, Kishimoto J, Liu L, Emson PC . Regional localization of the gene coding for human brain nitric oxide synthase (NOS1) to 12q24.2–24.31 by fluorescent in situ hybridization. Cytogenet Cell Genet 1993; 64:62–3.
Twell R, Xu W, Ball D, Baylin S, Allotey R, Williamson R, Chamberlain S . Exclusion of the neuronal nitric oxide synthase gene and the human achaete-scute homologue 1 gene as candidate loci for spinal cerebella ataxia 2. Am J Hum Genet 1995; 56:336–7.
Chung E, Curtis D, Chen G, Marsden PA, Twells R, Xu W . Gardiner M Genetic evidence for the neuronal nitric oxide synthase gene (NOS1) as a Susceptibility locus for infantile pyloric stenosis. Am J Hum Genet 1996; 58:363–70.
Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC . Targeted disruption of the neu- ronal nitric oxide synthase gene. Cell 1993 31; 75(7):1273–86.
Xu W, Charles I, Moncada S, Gorman P, Sheer D, Liu L, Emson PC . Mapping of the genes encoding human inducible and endothelial nitric oxide synthase (NOS2 and NOS3) to the peri- centric region of chromosome 17 and to chromosome 7, respectively. Genomics 1994; 21:419–22.
Xu W, Charles I, Moncada S., Gorman P, Sheer D, Liu L, Emson PC . Chromosomal assignment of the genes encoding for human neuronal, inducible and endothelial NO synthase: a dispersed gene family. The Biology of Nitric Oxide Part 4(Ed.Dr. Higgs, Portland Press Ltd. 1994:121–5.
Xu W, Charles I, Liu L, Koni P, Moncada S, Emson PC . Molecular genetic analysis of the duplication of human inducible nitric oxide synthesis (NOS2) sequences. Biochem Biophy Res Commun 1995; 212:466–72.
Xu W, Charles IG, Liu L, Moncada S, Emson P . Molecular cloning and structural organization of the human inducible nitric oxide synthase gene (NOS2). Biochem Biophys Res Commun 1996;219(3):784–8.
Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase Nature 1995; 377:239–42.
Wei XQ, Charles IG, Smith A, Ure J, Huang FP, Xu D, Muller W, Moncada S, Liew FY Altered immune responses in mice lacking inducible nitric oxide synthase Nature 1995; 375:408–11.
MacMicking JD, North RJ, Lacourse R, Mudgett JS, Shah SK, Nathan CF . Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA 1997; 94:5243–8.
Xu W, Liu L, Emson PC, Harrington CR, Charles IG . Evolution of a homopurine-homopyrimidine pentanucleotide repeats sequence upstream of the human inducible nitric oxide synthesis gene. Gene 1997; 204:165–70.
Burgner D, Xu W, Rockett K, Gravenor M, Charles IG, Hill AV, Kwiatkowski D . Inducible nitric oxide synthase polymorphism and fatal cerebral malaria. Lancet 1998;352(9135):1193–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, W., Liu, L. Nitric Oxide: from a mysterious labile factor to the molecule of the Nobel Prize. Cell Res 8, 251–258 (1998). https://doi.org/10.1038/cr.1998.25
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.1998.25
Keywords
This article is cited by
-
The apoptotic inducible effects of salicylic acid on hepatoma cell line: relationship with nitric oxide signaling
Journal of Cell Communication and Signaling (2017)
-
The eIF2α kinases: their structures and functions
Cellular and Molecular Life Sciences (2013)