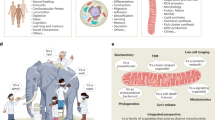
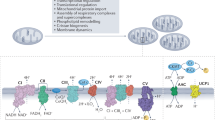
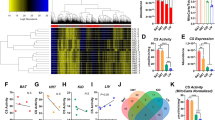
Abstract
Measurement of oxygen consumption is a powerful and uniquely informative experimental technique. It can help identify mitochondrial mechanisms of action following pharmacologic and genetic interventions, and characterize energy metabolism in physiology and disease. The conceptual and practical benefits of respirometry have made it a frontline technique to understand how mitochondrial function can interface with—and in some cases control—cell physiology. Nonetheless, an appreciation of the complexity and challenges involved with such measurements is required to avoid common experimental and analytical pitfalls. Here we provide a practical guide to oxygen consumption measurements covering the selection of experimental models and instrumentation, as well as recommendations for the collection, interpretation and normalization of data. These guidelines are provided with the intention of aiding experimental design and enhancing the overall reputability, transparency and reliability of oxygen consumption measurements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pagliarini, D. J. & Rutter, J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 27, 2615–2627 (2013).
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014).
Huang, S. C. C. et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. https://doi.org/10.1038/ni.2956 (2014).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature https://doi.org/10.1038/nature11986 (2013).
Choi, S. W., Gerencser, A. A. & Nicholls, D. G. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J. Neurochem. https://doi.org/10.1111/j.1471-4159.2009.06055.x (2009).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Chandel, N. S. Evolution of mitochondria as signaling organelles. Cell Metab. https://doi.org/10.1016/j.cmet.2015.05.013 (2015).
Murphy, M. P. & Hartley, R. C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. https://doi.org/10.1038/nrd.2018.174 (2018).
Pelletier, M., Billingham, L. K., Ramaswamy, M. & Siegel, R. M. Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol. https://doi.org/10.1016/B978-0-12-416618-9.00007-8 (2014).
Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim. Biophys. Acta 1807, 1507–1538 (2011).
Nicholls, D. G. & Ferguson, S. J. Bioenergetics 4 (Academic Press, 2013).
Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N. & Jastroch, M. Analysis and interpretation of microplate-based oxygen consumption and pH data. in Methods in Enzymology https://doi.org/10.1016/B978-0-12-801415-8.00016-3 (2014).
Doerrier, C. et al. High-resolution fluorespirometry and oxphos protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. in Methods in Molecular Biology https://doi.org/10.1007/978-1-4939-7831-1_3 (2018).
Will, Y., Hynes, J., Ogurtsov, V. I. & Papkovsky, D. B. Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes. Nat. Protoc. https://doi.org/10.1038/nprot.2006.351 (2007).
Perry, C. G. R., Kane, D. A., Lanza, I. R. & Neufer, P. D. Methods for assessing mitochondrial function in diabetes. Diabetes https://doi.org/10.2337/db12-1219 (2013).
Schmidt, C. A., Fisher-Wellman, K. H. & Neufer, P. D. From OCR and ECAR to energy: perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 297, 101140 (2021).
Brand, M. D. & Nicholls, D. G. Assessing mitochondrial dysfunction in cells. Biochem. J. https://doi.org/10.1042/BJ20110162 (2011).
Jones, A. E. et al. Forces, fluxes, and fuels: tracking mitochondrial metabolism by integrating measurements of membrane potential, respiration, and metabolites. Am. J. Physiol. 320, C80–C91 (2021).
Connolly, N. M. C. et al. Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. https://doi.org/10.1038/s41418-017-0020-4 (2018).
Dranka, B. P., Hill, B. G. & Darley-Usmar, V. M. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2010.01.015 (2010).
Rogers, G. W. et al. High-throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE https://doi.org/10.1371/journal.pone.0021746 (2011).
Divakaruni, A. S., Rogers, G. W. & Murphy, A. N. Measuring mitochondrial function in permeabilized cells using the Seahorse XF analyzer or a Clark-type oxygen electrode. Curr. Protoc. Toxicol. https://doi.org/10.1002/0471140856.tx2502s60 (2014).
Hynes, J., Swiss, R. L. & Will, Y. High-throughput analysis of mitochondrial oxygen consumption. in Methods in Molecular Biology https://doi.org/10.1007/978-1-4939-7831-1_4 (2018).
Acin-Perez, R., Benincá, C., Shabane, B., Shirihai, O. S. & Stiles, L. Utilization of human samples for assessment of mitochondrial bioenergetics: gold standards, limitation and future perspectives. Life 11, 949 (2021).
Hill, B. G. et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 393, 1485–1512 (2012).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2, 287–295 (2007).
Wieckowski, M. R. M. R., Giorgi, C., Lebiedzinska, M., Duszynski, J. & Pinton, P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 4, 1582–1590 (2009).
Kushnareva, Y. E., Wiley, S. E., Ward, M. W., Andreyev, A. Y. & Murphy, A. N. Excitotoxic injury to mitochondria isolated from cultured neurons. J. Biol. Chem. 280, 28894–28902 (2005).
Yang, K., Doan, M. T., Stiles, L. & Divakaruni, A. S. Measuring CPT-1-mediated respiration in permeabilized cells and isolated mitochondria. STAR Protoc. 2, 100687 (2021).
Benador, I. Y. et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885 (2018).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1303360110 (2013).
Salabei, J. K., Gibb, A. A. & Hill, B. G. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. https://doi.org/10.1038/nprot.2014.018 (2014).
Quintana, A., Kruse, S. E., Kapur, R. P., Sanz, E. & Palmiter, R. D. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl Acad. Sci. USA 107, 10996–11001 (2010).
Rich, P. R. & Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. https://doi.org/10.1042/BSE0470001 (2010).
Kiss, G. et al. The negative impact of α-ketoglutarate dehydrogenase complex deficiency on matrix substrate-level phosphorylation. FASEB J. 27, 2393–2406 (2013).
Ryan, D. G. et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. https://doi.org/10.1038/s42255-018-0014-7 (2019).
Gray, L. R. et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 22, 669–681 (2015).
Taylor, E. B. Functional properties of the mitochondrial carrier system. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2017.04.004 (2017).
Woodall, B. P. et al. Parkin does not prevent accelerated cardiac aging in mitochondrial DNA mutator mice. JCI Insight 5, e127713 (2019).
Norton, M. et al. ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics. Sci. Signal. 7, ra10 (2014).
Fu, Z. et al. Requirement of mitochondrial transcription factor A in tissue-resident regulatory T cell maintenance and function. Cell Rep. 28, 159–171 (2019).
Pagliarini, D. J. et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic β cells. Mol. Cell 19, 197–207 (2005).
Wang, G. et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with iPSC and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014).
Leonardi, R., Zhang, Y. M., Rock, C. O. & Jackowski, S. Coenzyme A: back in action. Prog. Lipid Res. https://doi.org/10.1016/j.plipres.2005.04.001 (2005).
Solmonson, A. & DeBerardinis, R. J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 293, 7522–7530 (2018).
Stefely, J. A. & Pagliarini, D. J. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci. 42, 824–843 (2017).
Daan Westenbrink, B. et al. Mitochondrial reprogramming induced by CaMKIIδ mediates hypertrophy decompensation. Circ. Res. 116, e28–e39 (2015).
Rotig, A. et al. Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 17, 215–217 (1997).
Diers, A. R., Broniowska, K. A., Chang, C. F., Hill, R. B. & Hogg, N. S-nitrosation of monocarboxylate transporter 1: inhibition of pyruvate-fueled respiration and proliferation of breast cancer cells. Free Radic. Biol. Med. 69, 229–238 (2014).
CHANCE, B. & WILLIAMS, G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 217, 409–427 (1955).
Estabrook, R. W. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol. 10, 41–47 (1967).
Ernster, L. & Schatz, G. Mitochondria: a historical review. J. Cell Biol. 91, 227–255 (1981).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128, 1487–1513 (2021).
Sun, H. & Wang, Y. Branched chain amino acid metabolic reprogramming in heart failure. Biochim. Biophys. Acta 1862, 2270–2275 (2016).
White, P. J. & Newgard, C. B. Branched-chain amino acids in disease. Science 363, 582–583 (2019).
Picard, M. et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 6, e18317 (2011).
Arruda, A. P. et al. Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 20, 1427–1435 (2014).
Nicholls, D. G. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem. Soc. Trans. https://doi.org/10.1042/BST0371385 (2009).
Adhihetty, P. J. et al. The role of PGC-1α on mitochondrial function and apoptotic susceptibility in muscle. Am. J. Physiol. Cell Physiol 297, C217–C225 (2009).
Agier, V. et al. Defective mitochondrial fusion, altered respiratory function, and distorted cristae structure in skin fibroblasts with heterozygous OPA1 mutations. Biochim. Biophys. Acta 1822, 1570–1580 (2012).
Clerc, P. & Polster, B. M. Investigation of mitochondrial dysfunction by sequential microplate-based respiration measurements from intact and permeabilized neurons. PLoS ONE 7, e34465 (2012).
Schulz, I. Permeabilizing cells: some methods and applications for the study of intracellular processes. Methods Enzymol. 192, 280–300 (1990).
Buescher, J. M. et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. https://doi.org/10.1016/j.copbio.2015.02.003 (2015).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell https://doi.org/10.1016/j.cell.2018.03.055 (2018).
Antoniewicz, M. R. A guide to 13C metabolic flux analysis for the cancer biologist. Exp. Mol. Med. https://doi.org/10.1038/s12276-018-0060-y (2018).
Divakaruni, A. S. & Brand, M. D. The regulation and physiology of mitochondrial proton leak. Physiology https://doi.org/10.1152/physiol.00046.2010 (2011).
Bertholet, A. M. & Kirichok, Y. Mitochondrial H+ leak and thermogenesis. Annu. Rev. Physiol. 84, 381–407 (2022).
Duchen, M. R. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 283, 41–50 (1992).
De Stefani, D., Rizzuto, R. & Pozzan, T. Enjoy the trip: calcium in mitochondria back and forth. https://doi.org/10.1146/annurev-biochem-060614-034216 (2016).
Villalobo, A. & Lehninger, A. L. Inhibition of oxidative phosphorylation in ascites tumor mitochondria and cells by intramitochondrial Ca2+. J. Biol. Chem. 255, 2457–2464 (1980).
Murphy, A. N., Bredesen, D. E., Cortopassi, G., Wang, E. & Fiskum, G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc. Natl Acad. Sci. USA. 93, 9893–9898 (1996).
Veliova, M. et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 21, e49634 (2020).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13, 890–901 (2014).
Leek, R., Grimes, D. R., Harris, A. L. & McIntyre, A. Methods: using three-dimensional culture (spheroids) as an in vitro model of tumour hypoxia. Adv. Exp. Med. Biol. 899, 167–196 (2016).
Simian, M. & Bissell, M. J. Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 216, 31–40 (2017).
De Graaf, I. A. M. et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 5, 1540–1551 (2010).
Lau, A. N. & Vander Heiden, M. G. Metabolism in the tumor microenvironment. Annu. Rev. Cancer Biol. 4, 17–40 (2020).
Kanow, M. A. et al. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6, e28899 (2017).
Harada, A. E., Healy, T. M. & Burton, R. S. Variation in thermal tolerance and its relationship to mitochondrial function across populations of Tigriopus californicus. Front. Physiol. 10, 213 (2019).
Luz, A. L., Smith, L. L., Rooney, J. P. & Meyer, J. N. Seahorse XFe24 extracellular flux analyzer-based analysis of cellular respiration in Caenorhabditis elegans. Curr. Protoc. Toxicol. 66, 25.7.1–25.7.15 (2015).
Lay, S., Sanislav, O., Annesley, S. J. & Fisher, P. R. Mitochondrial stress tests using seahorse respirometry on intact Dictyostelium discoideum cells. Methods Mol. Biol 1407, 41–61 (2016).
Muthusamy, T. et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 586, 790–795 (2020).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Tschöp, M. H. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2012).
Müller, T. D., Klingenspor, M. & Tschöp, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021).
Tyrrell, D. J. et al. Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Exp. Gerontol. 70, 84–91 (2015).
Kenwood, B. M. et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 3, 114–123 (2013).
Davila, A. et al. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. Elife 7, e33246 (2018).
Luongo, T. S. et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 (2020).
Affourtit, C. & Brand, M. D. Stronger control of ATP/ADP by proton leak in pancreatic beta cells than skeletal muscle mitochondria. Biochem. J. https://doi.org/10.1042/BJ20051280 (2006).
Krasnikov, B. F. et al. Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim. Biophys. Acta 1708, 375–392 (2005).
Mookerjee, S. A., Goncalves, R. L. S., Gerencser, A. A., Nicholls, D. G. & Brand, M. D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 1847, 171–181 (2015).
Desousa, B. R. et al. Calculating ATP production rates from oxidative phosphorylation and glycolysis during cell activation. Preprint at bioRxiv https://doi.org/10.1101/2022.04.16.488523 (2022).
Mookerjee, S. A., Gerencser, A. A., Nicholls, D. G. & Brand, M. D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. https://doi.org/10.1074/jbc.M116.774471 (2017).
Divakaruni, A. S., Andreyev, A. Y., Rogers, G. W. & Murphy, A. N. In situ measurements of mitochondrial matrix enzyme activities using plasma and mitochondrial membrane permeabilization agents. Anal. Biochem. 552, 60–65 (2018).
Divakaruni, A. S. et al. Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J. Cell Biol. https://doi.org/10.1083/jcb.201612067 (2017).
Grassian, A. R., Metallo, C. M., Coloff, J. L., Stephanopoulos, G. & Brugge, J. S. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 25, 1716–1733 (2011).
Sullivan, W. J. et al. Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell 175, 117–132 (2018).
Sessions, A. O. et al. Preserved cardiac function by vinculin enhances glucose oxidation and extends health- and life-span. APL Bioeng. 2, 036101 (2018).
Fan, Y. Y. et al. A bioassay to measure energy metabolism in mouse colonic crypts, organoids, and sorted stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 309, 1–9 (2015).
Taddeo, E. P. et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Mol. Metab. 16, 150–159 (2018).
Kooragayala, K. et al. Quantification of oxygen consumption in retina ex vivo demonstrates limited reserve capacity of photoreceptor mitochondria. Invest. Ophthalmol. Vis. Sci. 56, 8428–8436 (2015).
Ludikhuize, M. C., Meerlo, M., Burgering, B. M. T. & Rodríguez Colman, M. J. Protocol to profile the bioenergetics of organoids using Seahorse. STAR Protoc. 2, 100386 (2021).
Gerencser, A. A. et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. https://doi.org/10.1021/ac900881z (2009).
Oliver, D. G., Sanders, A. H., Douglas Hogg, R. & Woods Hellman, J. Thermal gradients in microtitration plates. Effects on enzyme-linked immunoassay. J. Immunol. Methods 42, 195–201 (1981).
Lundholt, B. K., Scudder, K. M. & Pagliaro, L. A simple technique for reducing edge effect in cell-based assays. J. Biomol. Screen. 8, 566–570 (2003).
Schoonen, W. G. E. J., Stevenson, J. C. R., Westerink, W. M. A. & Horbach, G. J. Cytotoxic effects of 109 reference compounds on rat H4IIE and human HepG2 hepatocytes. III: Mechanistic assays on oxygen consumption with MitoXpress and NAD(P)H production with Alamar Blue. Toxicol. In Vitro 26, 511–525 (2012).
Little, A. C. et al. High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions. Commun. Biol. 3, 271 (2020).
Wiley, S. E. et al. Wolfram syndrome protein, Miner1, regulates sulphydryl redox status, the unfolded protein response, and Ca2+ homeostasis. EMBO Mol. Med. 5, 904–918 (2013).
Wettmarshausen, J. & Perocchi, F. Isolation of functional mitochondria from cultured cells and mouse tissues. Methods Mol. Biol. 1567, 15–32 (2017).
Kirkinezos, I. G. et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J. Neurosci. 25, 164–172 (2005).
Rolfe, D. F. S. & Brown, G. C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 (1997).
Chacko, B. K. et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes and neutrophils, and the oxidative burst from human blood. Lab. Invest. 93, 690–700 (2013).
van der Windt, G. J. W. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
McMurray, J. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
Molina, J. R. et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 24, 1036–1046 (2018).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Nowinski, S. M. et al. Mitochondrial fatty acid synthesis coordinates oxidative metabolism in mammalian mitochondria. Elife 9, e58041 (2020).
Floyd, B. J. et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol. Cell 63, 621–632 (2016).
Brand, M. D. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 33, 897–904 (2005).
Watt, I. N., Montgomery, M. G., Runswick, M. J., Leslie, A. G. W. & Walker, J. E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl Acad. Sci. USA 107, 16823–16827 (2010).
Hinkle, P. C. P/O ratios of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta 1706, 1–11 (2005).
Müller, T. D. et al. P62 links β-adrenergic input to mitochondrial function and thermogenesis. J. Clin. Invest. 123, 469–478 (2013).
Kory, N. et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv 6, 43 (2020).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila and humans. Science https://doi.org/10.1126/science.1218099 (2012).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science https://doi.org/10.1126/science.1218530 (2012).
Sharma, A. et al. Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole-body leanness. Elife 8, e45873 (2019).
Bertholet, A. M. The use of the patch-clamp technique to study the thermogenic capacity of mitochondria. J. Vis. Exp. 171, (2021).
Gerencser, A. A. et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. https://doi.org/10.1113/jphysiol.2012.228387 (2012).
Acknowledgements
A.S.D. is supported by National Institutes of Health grants R35GM138003, P30DK063491 and P50CA092131, as well as the W. M. Keck Foundation. M.J. is supported by the Novo Nordisk Research Fonden (NNF20OC0059646). We thank members of both of our laboratories for their helpful discussions during the preparation of this manuscript, as well as L. Stiles (UCLA), B. Desousa (UCSF) and A. Murphy (Cytokinetics) for their critical perspective.
Author information
Authors and Affiliations
Contributions
A.S.D. and M.J. both contributed to the conceptualization, preparation, writing and editing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no current competing interests. A.S.D. has previously served as a paid consultant for Agilent Technologies.
Peer review
Peer review information
Nature Metabolism thanks Alexander Galkin, David Nicholls and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Divakaruni, A.S., Jastroch, M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat Metab 4, 978–994 (2022). https://doi.org/10.1038/s42255-022-00619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00619-4
This article is cited by
-
NDUFS4 regulates cristae remodeling in diabetic kidney disease
Nature Communications (2024)