Abstract
CD8+ T-cell functions are critical for preventing chronic viral infections by eliminating infected cells. For healthy immune responses, beneficial destruction of infected cells must be balanced against immunopathology resulting from collateral damage to tissues. These processes are regulated by factors controlling CD8+ T-cell function, which are still incompletely understood. Here, we show that the interferon regulatory factor 4 (IRF4) and its cooperating binding partner B-cell-activating transcription factor (BATF) are necessary for sustained CD8+ T-cell effector function. Although Irf4–/– CD8+ T cells were initially capable of proliferation, IRF4 deficiency resulted in limited CD8+ T-cell responses after infection with the lymphocytic choriomeningitis virus. Consequently, Irf4–/– mice established chronic infections, but were protected from fatal immunopathology. Absence of BATF also resulted in reduced CD8+ T-cell function, limited immunopathology, and promotion of viral persistence. These data identify the transcription factors IRF4 and BATF as major regulators of antiviral cytotoxic T-cell immunity.
Similar content being viewed by others
Main
Chronic viral infections such as those involving the hepatitis C virus and the human immunodeficiency virus (HIV) represent a major global health burden.1, 2 Control of viral infections requires effective CD8+ T-cell responses to kill virus-infected cells. However, destruction of infected cells in an attempt to control viral replication can damage tissues and even result in death. Identifying factors and mechanisms involved in modulating cytotoxic T-cell functions is critical for providing novel opportunities to treat chronic infections.
The interferon regulatory factor 4 (IRF4) is an important immunological transcription factor required for B-cell maturation3 and for the maintenance of various CD4+ T-cell subsets including T helper (Th2),4, 5 regulatory T cell,6 Th9,7, and Th178 cells. However, the role of IRF4 in CD8+ T-cell responses is less clear. IRF4 can influence the differentiation of CD8+ T cells,9 including a subset that produces interleukin (IL)-17.10 Cytotoxic responses towards the lymphocytic choriomeningitis virus (LCMV) are also reduced during IRF4 deficiency,3 although it is unclear whether this defect is due to CD8+ T cells or due to additional cell types. Very recently, it has been reported that IRF4 is critical for CD8+ T-cell responses to Listeria monocytogenes11 and for maintenance of effector CD8+ T-cell function in response to the influenza virus.12, 13
IRF4 binds cooperatively with an activator protein-1 (AP-1) family heterodimer consisting of B-cell-activating transcription factor (BATF) and Jun-regulating gene expression in CD4+ T cells during Th17 cell specification.14, 15, 16 Phenotypes for BATF- and IRF4-deficient B and T cells are similar,17 and there is emerging evidence that BATF is also necessary for functional CD8+ T-cell responses. For instance, absence of BATF results in fewer effector CD8+ T cells directed against model antigen ovalbumin (OVA).18 However, the function of BATF in an in vivo model of viral infection has not yet been investigated, and its role during antiviral T-cell immunity remains unclear.
In this study, we found that IRF4 and BATF were dispensable for initial T-cell proliferation but that absence of IRF4 or BATF resulted in limited T-cell numbers and function following infection with LCMV. Consequently, Irf4–/– mice showed reduced immunopathology but were less able to control viral replication. Consistently, BATF deficiency resulted in limited immunopathology, reduced cytotoxic T-cell immunity, and viral persistence after infection with LCMV.
Results
IRF4 deficiency inhibits T-cell-mediated hepatitis
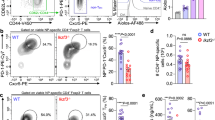
We first examined the response of wild-type (WT) and Irf4−/− mice to LCMV infection, which itself is not cytopathic, but where liver damage occurs as a consequence of virus-specific T-cell activity.19 Absence of IRF4 during infection resulted in reduced quantities of virus-specific (gp33-tet+) CD8+ T cells relative to WT mice (Figure 1a). Consistently, restimulation of splenocytes with LCMV peptides glycoprotein (gp33) or nucleoprotein (np396) resulted in less activated interferon-γ (IFN-γ)-producing CD8+ T cells in samples harvested from Irf4−/− compared with WT animals (Figure 1b). Control of LCMV largely depends on CD8+ T cells to eliminate infected cells. Consistently, infectious particles could be detected in the spleen, liver, lung, and kidney tissue harvested from Irf4−/− mice at 12 and 20 days post infection, whereas significantly lower or undetectable levels were present in organs from WT mice (Figure 1c). During viral infections, activated CD8+ T cells can cause collateral damage to tissues even during infections with viruses possessing low levels of intrinsic cytotoxicity.1, 2, 20 Accordingly, in the model of LCMV used here, liver damage is known to be driven by CD8+ T cells.19 Consistent with the reduced presence of virus-specific and cytokine-producing CD8+ T cells, infected Irf4−/− mice were resistant to infection-associated liver damage observed in WT mice by release of the liver-specific enzyme alanine aminotransferase (ALT) into the serum (Figure 1d). These effects may be further explained by reduced lymphocyte infiltration seen in hematoxylin and eosin-stained liver sections from infected Irf4−/− mice compared with Irf4+/+ mice (Figure 1e). Thus, the absence of IRF4 resulted in reduced CD8+ T-cell function, limited control of viral replication, and resistance to T-cell-mediated immunopathology in vivo.
IRF4 deficiency impairs control of LCMV replication and prevents induction of T cell-mediated immunopathology. (a–e) Irf4+/+or Irf4−/− mice were infected with 2 × 106 PFU LCMV-WE. (a) Proportion of gp33-H2-Db (gp33) and np396-H2-Db (np396) tet+ CD8+ T cells in single cell-suspended spleen tissue from WT and IRF4-deficient mice 12 days after infection was analysed, and (b) Quantity of CD8+ T cells harvested 12 days after infection capable of producing IFN-γ following in vitro restimulation with gp33 or np396 is shown (flow cytometry; % of CD8+ cells; mean +/− S.E.M., n=6 mice/group from two pooled experiments). (c) Virus titers were determined in spleen, liver, lung, and kidney tissues on day 12 (D12) and 20 (D20) postinfection in WT and IRF4-deficient mice (mean±S.E.M., n=5–7 mice/group). (d) Liver damage was assessed by measuring ALT activity in the sera of WT and IRF4-deficient mice at different time points after infection as indicated (mean±S.E.M., n=4 mice/group). (e) Snap-frozen liver sections from WT and IRF4-deficient animals were stained with hematoxylin and eosin. One representative image from n=4 mice/group is shown (scale bar, 200 μm)
IRF4-deficient T cells have defects during in vitro culture
We next investigated whether defects would also be present in IRF4-deficient CD8+ T cells cultured in vitro. IRF4 protein expression in CD8+ T cells was strongly upregulated by the presence of anti-CD3 antibodies in the culture medium (Figure 2a). Although both WT and Irf4−/− CD8+ T cells actively proliferated following T-cell receptor (TCR) stimulation in vitro (Figure 2b and Supplementary Figure 1), fewer Irf4−/− CD8+ T cells were observed following 72 h of culture compared with WT (Figure 2c). Relative to WT, Irf4−/− CD8+ T cells were less able to produce cytokines associated with effector function (IFN-γ) and survival (IL-2) (Figures 2d and e). Furthermore, a larger proportion Irf4−/− CD8+ T cells appeared to be undergoing apoptosis based on elevated ceramide staining (Figure 2f) and increased expression of activated caspase 3 (Figure 2g). Active caspase 3 levels could be reduced in both genotypes by the addition of the pan-caspase inhibitor Q-VD-OPh (QVD) to the culture medium (Figure 2g). Consistently, a lower percentage of viable cells (determined by annexin V (AV) and 7-aminoactinomycin D (7-AAD) exclusion) was observed in vitro in the absence of IRF4, an effect that could be partially rescued by the addition of QVD (Figure 2h). These data suggest that Irf4−/− CD8+ T cells have defects in vitro, including elevated apoptosis, which may be one factor contributing to the reduced overall quantity of effector CD8+ T cells observed in the IRF4-deficient setting.
CD8+ T cells from Irf4−/− mice have defects in vitro. (a) Negatively sorted CD8+ T cells from WT mice were cultured for 96 h with 5 μg/ml antiCD3. IRF4 expression was measured by intracellular staining and flow cytometry with mean fluorescence intensity (left)(mean±S.E.M.; n=3, one out of two independent experiments shown), and by western blot with actin loading control (right)(one representative of n=3 experiments is shown). (b) Cells from Irf4+/+ versus Irf4−/− mice were stimulated in vitro with, or without (control), 5 μg/ml antiCD3 and 2 μg/ml antiCD28 for 72 h and CFSE fluorescence was determined by flow cytometry. Representative histograms are shown of n=7. (c) Absolute cell count from b (mean +/− S.E.M.; n=7). (d–h) Negatively sorted Irf4+/+ versus Irf4−/− T cells were stimulated with medium alone (control) or 5 μg/ml antiCD3 and 2 μg/ml antiCD28, plus Q-VD-OPh (QVD) where indicated, for 72 h in vitro. (d) Intracellular staining for IFN-γ by flow cytometry is displayed (mean±S.E.M., n=6). (e) IL-2 concentration in conditioned media was measured by ELISA and mean±S.E.M. is illustrated (n=6). (f) Cell surface ceramide staining was assessed by flow cytometry (mean±S.E.M., n=6). (g) Intracellular staining for active caspase 3 was determined by flow cytometry (mean±S.E.M., n=6). (h) Cells were exposed to AV and 7-AAD followed by analysis with flow cytometry. Representative dot plots (8000 events displayed/plot)(left) of n=6 are shown, as well as % viable cells (AV−, 7-AAD−, mean ±S.E.M., n=6, right)
IRF4 deficiency impacts response to low doses of LCMV
In order to investigate the effects of IRF4 deficiency on CD8+ T cells more closely, we next infected WT or Irf4−/− mice with a lower dose of LCMV, where substantial quantities of virus-specific CD8+ T cells persisted in both genotypes. Virus-specific CD8+ T cells were detected in Irf4−/− mice at 8 days post infection, although in lower levels than in controls (for gp33-tet+ cells) (Figure 3a). Moreover, CD8+ T cells isolated from both groups were capable of producing IFN-γ after restimulation with LCMV peptides gp33 or np396, but less cytokine production was observed in the absence of IRF4 (Figure 3b). Even after adjusting for differences in the number of virus-specific CD8+ T cells, less cytotoxicity was observed on a per-cell basis in the Irf4−/− setting relative to the WT setting after LCMV infection (Figure 3c). Interestingly, although cytotoxicity was readily apparent in IRF4-deficient T cells 8 days after infection, it appeared to be further reduced by day 10 (Supplementary Figure 2), suggesting that IRF4-deficient T cells may exhibit progressive loss of T-cell functionality. Furthermore, similar to our observations in vitro, a larger proportion of virus-specific IRF4-deficient CD8+ T cells appeared to be undergoing apoptosis compared with WT controls based on AV and 7-AAD staining (Figure 3d).
Cytotoxic CD8+ T-cell responses to low-dose LCMV are impaired in the absence of IRF4 relative to controls. (a–d) Irf4+/+or Irf4−/− mice were infected with 200 PFU of LCMV-WE. (a) Percentage of gp33- and np396-tet+ CD8+ T cells in spleen tissue was measured 8 days after infection (% of CD8+ T cells, n=4, one of three independent experiments is shown). (b) Percentage of CD8+ T cells 8 days after infection capable of producing IFN-γ after restimulation with virus-specific peptides gp33 or np396 was measured by intracellular staining and flow cytometry (n=8, pooled from three independent experiments). (c) Eight days after infection, splenocytes from WT and IRF4-deficient mice were harvested and incubated with 51Cr labeled-EL-4 cells. Peptide-specific 51Cr release is shown in % for indicated effector/target ratios (gp33-tet+ CD8+ cells/EL-4 cells)(mean±S.E.M., measured in duplicate, n=4–5). (d) Cell viability was determined by ex vivo staining with AV (AV) and 7-AAD measured on gp33-tet+ CD8+ T cells from spleen tissue of Irf4+/+or Irf4−/− mice 8 days post infection (mean±S.E.M., n=4 mice/group)
IRF4-deficient CD8+ T cells fail to induce immunopathology
In order to study the CD8+ T-cell-specific effects of IRF4 without the potential influence of other T-cell lineages, Irf4−/− mice were first crossed onto the P14 transgenic background. P14 mice express a transgenic TCR recognizing the LCMV gp33 epitope.21 Negatively sorted CD8+ T cells from Irf4+/+ or Irf4−/− P14 transgenic TCR mice were then transferred into recombination-activating gene 1 (Rag1–/–) mice22 1 day before infection with a chronic strain of LCMV (clone 13). Both Irf4+/+ and Irf4−/− P14 transfer resulted in expansion of virus-specific CD8+ T cells measured in blood from recipients between 0 and 6 days post infection; however, fewer virus-specific T cells were observed in the absence of IRF4 by days 4 and 6 (Figure 4a). Consistently, after restimulation with gp33, IFN-γ+ CD8+ T cells were seen to be markedly reduced on days 4 and 6 in blood samples from recipients of Irf4−/−P14 cells, compared with recipients of Irf4+/+ P14 cells (Figure 4b). Strong CD8+ T-cell responses can result in fatal immunopathology after chronic LCMV infection;23 therefore, we examined the survival of infected mice receiving either transfer of Irf4+/+ or Irf4−/− P14 T cells. Recipients of Irf4+/+ P14 cells rapidly succumbed to severe immunopathology within ∼8 days, whereas recipients of Irf4−/−P14 cells survived the length of the observation period (Figure 4c). Thus, in this context, CD8+ T-cell-specific defects limit the expansion of virus-specific cells and block the induction of fatal immunopathology in the absence of IRF4.
Transferred Irf4−/− CD8+ T cells fail to induce immunopathology. (a–c) 3 × 106 negatively sorted CD8+ T cells from CD45.1+Irf4+/+P14 or CD45.1+Irf4−/− P14 mice were transferred into Rag1–/– mice one day before infection with 2 × 106 PFU LCMV clone13. (a) Quantity of transferred gp33 tetramer-specific CD8+ T cells was measured in the blood stream at indicated days following infection (quantity/10 μl blood, mean±S.E.M., n=6–9 mice/group). (b) Amount of IFN-γ+CD8+CD45.1+T cells after restimulation with virus-specific peptide gp33 was quantified (quantity/10 μl blood, mean±S.E.M., n=5–6 mice/group). (c) Survival of infected mice, which received negatively sorted Irf4+/+ versus Irf4−/− P14 T cells was monitored overtime (n=6 mice/group)
Impaired Irf4−/− T-cell function results in limited memory responses
We next investigated whether defects in CD8+ T-cell responses observed in the absence of IRF4 would extend to the memory phase. Similar to observations at earlier time points (Figures 1 and 3), at both 30 and 60 days following infection with LCMV, reduced quantities of virus-specific (gp33-tet+) T cells were observed in the spleen, bone marrow, and lymph nodes of IRF4-deficient mice compared with WT animals (Figure 5a). At the same time points, virus-specific CD8+ T cells from Irf4−/− mice had altered surface expression of CD27, CD44, CD62L, interleukin-7 receptor, and killer cell lectin-like receptor subfamily G member 1 (KLRG1) compared with cells from Irf4+/+ mice, with particularly low expression of KLRG1 in the absence of IRF4 (Supplementary Figure 3). Restimulation with the LCMV gp33 peptide in vitro resulted in robust IFN-γ, tumor necrosis factor-α, and IL-2 cytokine production in CD8+ T cells isolated from Irf4+/+ mice, but not from Irf4−/− mice, at both 30 and 60 days post infection (Figure 5b). Next, we measured persistence of transferred Irf4+/+ versus Irf4−/− P14 CD8+ T cells in WT mice following LCMV infection. At both 30 and 60 days post infection, massively reduced quantities of Irf4−/−-transferred virus-specific T cells were detectable relative to Irf4+/+ controls (Figure 5c). Finally, we tested the ability of transferred WT or IRF4-deficient P14 T cells to induce a memory CD8+ T-cell immune response in WT animals infected initially with LCMV and subsequently challenged with a recombinant strain of Vaccinia virus expressing an LCMV-GP.24 Following secondary challenge, mice receiving WT P14 T cells displayed overt cytotoxicity to EL4 cells coated with P14-specific peptides, whereas no cytotoxicity was detected for mice receiving IRF4-deficient P14 T cells (Figure 5d). Taken together, these data suggest that IRF4 deficiency results in reduced CD8+ effector function and inability to produce normal CD8+ memory responses.
LCMV memory responses are diminished in the absence of IRF4. (a–c) Irf4+/+or Irf4−/− mice were infected with 2 × 105 PFU LCMV-ARM. (a) Amount of gp33-tet+ CD8+ T cells was measured in the spleen, bone marrow (BM) and lymph node (LN) 30 days (D30) and 60 days (D60) postinfection (mean±S.E.M., n=4 mice/group), (b) Quantity of CD8+ T cells harvested 30 (D30) or 60 (D60) days after infection capable of producing IFN-γ, TNFα or IL-2 following restimulation with virus-specific peptide gp33 was evaluated (flow cytometry; % of CD8+ cells; mean±S.E.M., n=4 mice/group). (c) 4 × 104 isolated CD8+ T cells from CD45.1+Irf4+/+P14 or CD45.1+Irf4−/−P14 mice were transferred into CD45.2+ WT mice one day before infection. Total quantity of gp33 tetramer-specific CD45.1+CD8+ T cells in the spleen, BM and LN 30 (D30) or 60 (D60) days postinfection were measured (mean±S.E.M., n=5 mice/group). (d) WT mice receiving 2 × 105 Irf4+/+ or Irf4−/− P14 CD8+ T cells on day 1 were infected with 2 × 103 PFU LCMV-ARM on day 0 and rechallenged with 2 × 106 PFU Vaccinia-gp 8.5 months later (on day 254). Three days after infection with Vaccinia-gp, splenocytes were harvested and incubated with MB6 peptide loaded 51Cr labeled-EL-4 cells. Peptide-specific EL-4 cell lysis was calculated in % for indicated effector/target ratios (mean±S.E.M., measured in duplicate, n=3 mice/group)
The IRF4 binding partner BATF is critical for control of LCMV
IRF4 functionally cooperates with AP-1 transcription factor heterodimer BATF–Jun in binding to composite elements in T cells.15, 17 Because BATF has been associated with reduced T-cell immunity,18 we wondered whether mice lacking BATF25 might have defects similar to IRF4-deficient mice in controlling viral infection. Following TCR stimulation, expression of BATF was strongly upregulated during in vitro culture of WT CD8+ T cells (Figure 6a). WT and Batf−/− CD8+ T cells displayed similar proliferative capacity in vitro (Figures 6b and c and Supplementary Figure 4). However, when BATF-deficient mice were challenged with low-dose LCMV, reduced levels of virus-specific CD8+ T cells were present compared with WT mice (Figure 6d). Consistently, Batf–/– CD8+ T cells from infected mice also failed to produce large quantities of IFN-γ after stimulation with LCMV peptides in sharp contrast to CD8+ T cells from WT animals (Figure 6e). Similar to observations during IRF4 deficiency, a larger proportion of virus-specific BATF-deficient CD8+ T cells appeared to be undergoing apoptosis relative to cells harvested from WT animals (Figure 6f). Consistent with impaired maintenance of a normal population of virus-specific T cells, BATF-deficient mice failed to control viral replication in the spleen, liver, and lung tissue 8 days after infection, whereas virus was not detectable in the organs of WT animals (Figure 6g). Therefore, healthy immune responses to control LCMV infection require BATF.
The transcription factor BATF is necessary for responses to LCMV. (a) BATF protein expression in negatively sorted WT CD8+ T cells cultured for 96 h with 5 μg/ml antiCD3. BATF expression was determined by intracellular staining followed by flow cytometry analysis (left) (means±S.E.M.; n=9). BATF expression was further assessed by western blot shown with actin loading control (right)(one representative of n=3 experiments is shown). (b) Negatively sorted, CFSE labeled Batf +/+ and Batf−/− T cells were stimulated in vitro with, or without (control), 5 μg/ml antiCD3 and 2 μg/ml antiCD28 for 72 h and CFSE expression was measured by flow cytometry. Representative histograms of n=7 are shown. (c) Quantification of total number of cells from b is illustrated (mean±S.E.M.; n=7 mice/group). (d–g) Batf+/+and Batf −/− mice were infected with 200 PFU LCMV-WE. (d) Quantity of gp33- and np396- tetramer+ CD8+ T cells in the spleen 8 days after infection was measured (% of total CD8+ T cells, mean±S.E.M., n=6 mice/group). (e) Quantity of IFN-γ producing CD8+ T cells 8 days after infection following restimulation with virus-specific peptides gp33 or np396 was assessed (mean±S.E.M., n=6 mice/group). (f) Cell viability was determined ex vivo by staining with Annexin V (AV) and 7-AAD on gp33 tetramer-specific CD8+ T cells from Batf+/+or Batf −/− mice on day 8 postinfection and analysis with flow cytometry (mean±S.E.M., n=7 mice/group). (g) LCMV titers in spleen, liver, lung, and kidney tissue from infected Batf+/+ or Batf−/− mice are shown on day 8 post infection (mean±S.E.M., n=6 mice/group)
BATF deficiency results in CD8+ T-cell-specific defects
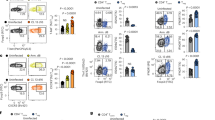
Next, we addressed whether the defects observed in BATF-deficient animals might be connected to limited CD8+ T-cell immunity. We therefore tested whether transfer of WT P14 T cells could rescue virus control in Batf–/– mice. When WT virus-specific CD8+ P14 T cells were transferred into Batf+/+ or Batf–/– mice, they showed equivalent expansion and ability to produce IFN-γ after infection in both settings (Figures 7a and b). Moreover, WT P14 transfer almost entirely rescued defective virus control associated with the absence of BATF (Figure 7c). These data suggested that observed defects in viral control in the absence of BATF were likely a consequence of reduced CD8+ T-cell function. In order to more directly assess CD8+ T-cell function in the absence of BATF, we crossed BATF mice with P14 transgenic TCR mice21 and performed a similar experiment to that shown for IRF4 in Figure 4. Briefly, negatively sorted CD8+ T cells from Batf+/+ or Batf−/− P14 transgenic TCR mice were transferred into Rag1–/– mice 1 day before infection with a chronic strain of LCMV (clone 13). Both transferred Batf+/+ or Batf−/− P14 CD8+ T cells expanded between 0 and 6 days post infection; however, in the absence of BATF, less virus-specific cells were observed on day 6 post infection (Figure 7d). Consistently, restimulation with gp33 resulted in fewer IFN-γ-producing cells in the blood from recipients of Batf−/− compared with Batf+/+ P14 cells (Figure 7e). Similar to observations during IRF4 deficiency (Figure 4c), recipients of Batf+/+ P14 cells rapidly succumbed to severe immunopathology within ∼8 days, whereas recipients of Batf−/− P14 cells survived the length of the observation period (Figure 7f). Thus, BATF deficiency appears to result in CD8+ T-cell intrinsic defects that block the induction of fatal immunopathology.
Control of LCMV and induction of immunopathology depends on BATF expression in CD8+ T cells. (a–c) 2 × 105 splenocytes from WT CD45.1+P14 mice were transferred into Batf+/+ or Batf−/− mice one day before infection with 200 PFU LCMV-WE. (a) Quantity of gp33-tet+CD45.1+CD8+ T cells in spleen tissue from WT and BATF-deficient animals 8 days after infection is shown (mean±S.E.M., n=5 mice/group). (b) Amount of restimulated IFN-γ producing CD45.1+CD8+ T cells in spleen tissue from Batf+/+ and Batf–/– mice 8 days after infection as measured following restimulation with the virus-specific peptide gp33 by intracellular staining and flow cytometry is shown (mean±S.E.M., n=5). (c) LCMV virus titers in spleen, liver, lung, and kidney tissues of infected Batf+/+ or Batf−/− mice in the presence or absence of WT P14 transfer was measured (mean±S.E.M., n=3–5 mice/group). (d–f) 3 × 106 negatively sorted CD8+ T cells from Batf+/+ P14 or Batf−/− P14 mice were transferred on day -1 into Rag1–/– mice followed by infection with 2 × 106 PFU of LCMV Clone13. (d) Quantity of transferred gp33-tetramer+ CD8+ T cells measured in the blood at indicated days following infection is shown (quantity/10 μl blood, mean±S.E.M., n=4 mice/group). (e) Amount of IFN-γ+CD8+ T cells after restimulation with the virus-specific peptide gp33 was measured (quantity/10 ul blood, mean±S.E.M., n=4 mice/group). (f) Survival of infected mice that received Batf+/+ versus Batf−/− P14 T cells was monitored over time (n=4 mice/group)
Discussion
This study establishes an important role for both IRF4 and BATF in the maintenance of CD8+ T-cell responses to LCMV. After infection, both IRF4- and BATF-deficient mice showed reduced quantities of virus-specific and cytokine-producing T cells, and suffered from longer persistence of LCMV infections.
Although IRF4-deficient mice were less able to control viral replication, reduced CD8+ T-cell responses meant that, during acute LCMV infection, IRF4-deficient mice were protected from T-cell-driven hepatic immunopathology (Figure 1). This effect appears to be CD8+ T-cell intrinsic, and independent of defects in other T-cell lineages,4, 5, 6, 7, 8 as transfer of P14 CD8+ T cells into Rag1−/− mice (which lack mature B and T cells) resulted in fatal LCMV immunopathology in the presence, but not in the absence, of IRF4 (Figure 4). Thus, in this context, lacking IRF4 can protect from toxic immunopathology, but at the expense of uncontrolled viral replication, which may be catastrophic in other settings – for instance, those involving infection with cytopathic viruses.
Consistent with our observations during LCMV infection, recent reports further establish a necessity for IRF4 in effector CD8+ T-cell functions towards L. monocytogenes and influenza virus.11, 12 Interestingly, although we observe that initial expansion of IRF4-deficient T cells is evident both in vitro and in vivo (Figures 2b and 4a), quantities of virus-specific T cells are markedly reduced at later time points after LCMV infection. Furthermore, there is a trend towards declining cytotoxicity between days 8 and 10 following LCMV infection, specifically in the IRF4-deficient setting (Figure 3c and Supplementary Figure 2). These results suggest that CD8+ effector function may progressively decline in the absence of IRF4 and are consistent with observations of progressive loss of CD8+ effector function after influenza virus infection.12 Furthermore, in the absence of IRF4, reduced CD8+ effector function remained evident at later time points after LCMV infection, and cytotoxic recall responses to a related secondary infection were blocked (Figure 5). These data are also consistent with impaired memory cell formation observed in the absence of IRF4 after infection with L. moncytogenes.11
One potential mechanism contributing to reduced CD8+ effector function that is supported by both our data and two recent studies12, 13 appears to be enhanced cell death of Irf4−/− virus-specific T cells (Figures 2f–h and 3d). Similar to these studies, we observed that, in vitro, IRF4-deficient T cells did not expand to the same degree as WT controls, and displayed reduced viability and elevated active caspase 3 staining consistent with apoptosis.26 Cell death is a key mechanism limiting effector T-cell responses,27 including following LCMV infection.28 However, the contribution of other potential factors to limited CD8+ T-cell effector function, including abnormalities in metabolic reprogramming,12 defects in effector T-cell differentiation,13 or T cell exhaustion,29 warrants investigation in future studies.
Elevated cell death may also occur in BATF-deficient virus-specific CD8+ T cells (Figure 6f),13 suggesting that similar defects might govern reduced CD8+ effector function in both IRF4- and BATF-deficient mice. BATF deficiency resulted in reduced ability to respond to infection with LCMV and virus persistence (Figure 6). These results are consistent with a reduction in CD8+ effector T cells observed in BATF-deficient mice after immunization with the model antigen OVA.18 Furthermore, defects in viral control in Batf–/– mice could be overcome by transfer of WT P14 CD8+ T cells (Figures 7a–c), suggesting that inability to control LCMV infection in the absence of BATF is likely due to problems in the CD8+ T-cell compartment. Similar to observations during IRF4 deficiency (Figure 4), and in contrast to WT, absence of BATF in transferred virus-specific CD8+ P14 T cells precluded the induction of fatal T-cell immunopathology following infection of Rag1−/− mice (Figures 7d–f). Therefore, BATF deficiency appears to result in T-cell intrinsic defects that restrict effector function towards LCMV. Interestingly, BATF may be relevant for exhaustion of virus-specific CD8+ T cells during HIV infection in humans.30 Future studies will aim to clarify how this function relates to our observations and those of Kuroda et al.,18 which link absence of BATF to reduced CD8+ T-cell function in mice.
Our results extend previous knowledge of the involvement of IRF4 in the differentiation of certain CD8+ T-cell populations9, 10 and indicate that IRF4-deficient cytotoxic CD8+ T cells have an additional block in effector function evident after LCMV infection. We observed similar defects in T-cell immunity and virus control in the absence of either IRF4 or BATF, suggesting that functional cooperation of BATF and IRF4 reported for other T-cell subsets17 is likely also necessary for normal CD8+ T-cell function. In conclusion, we identified IRF4 and BATF to be critical regulators for CD8+ T-cell function during viral infection. Mechanisms underlying the control of these factors over CD8+ T-cell activity warrant future investigation to uncover novel opportunities for therapeutic intervention during treatment of chronic viral infections in humans.
Materials and Methods
Mice, viruses, and virus titration
All mice used in this study were maintained on a C57BL/6-J genetic background. IRF4-deficient, BATF-deficient, P14, and Rag1-deficient mice have been previously described.3, 21, 25 Histological analysis of snap-frozen liver tissue was performed as previously described.31 The LCMV-WE strain (originally obtained from F. Lehmann-Grube, Heinrich Pette Institute, Hamburg, Germany), LCMV Clone13 (a gift from Sam Basta, Queens University), and LCMV Armstrong (ARM) (provided by R.M. Zinkernagel, University of Zurich) were propagated in L929 cells as previously described.32 Virus titers were measured using a plaque-forming assay also as previously described.32 Mice were infected intravenously with 200 or 2 × 106 plaque-forming units (PFU) LCMV-WE, 2 × 106 PFU LCMV clone13, or 2 × 103 or 2 × 105 PFU LCMV Armstrong. Following infection, ALT values in serum were measured as previously described.33 All experiments were performed in single ventilated cages. Animal experiments were carried out in accordance with the guidelines of the Ontario Cancer Institute and the German law for animal protection.
Purification of T cells
CD8+ or total T-cell populations were purified from single cell-suspended splenocytes by negative selection following the manufacturer’s instructions (mouse CD8a+ T cell Isolation Kit II, mouse Pan T Cell Isolation Kit II, Miltenyi, Bergisch Gladbach, Germany).
In vitro T-cell culture
Purified T cells were cultured in RPMI 1640 culture medium (Biochrom, Berlin, Germany) with supplements (10% FCS, Biochrom) in 96-well flat-bottom tissue culture plates precoated with 5 μg/ml anti-CD3 (eBioscience, San Diego, CA, USA), 2 μg/ml soluble anti-CD28 (BD Pharmingen, Franklin Lakes, NJ, USA) (2 × 105 cells/well), and 10 μM Q-VD-OPh hydrate (Sigma, St. Louis, MO, USA) where indicated. Cells were cultured at 37 °C in a humidified 5% CO2 incubator for 72 h. For proliferation analysis, isolated T cells were labeled with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA, USA) for 10 min at 37 °C and washed twice with culture medium.
Transfer of P14 T cells
CD8+ T cells were isolated by negative selection (mouse CD8a+ T cell Isolation Kit II,mouse Pan T Cell Isolation Kit II, Miltenyi) from Irf4−/−, Batf−/−, or P14 mice as indicated, and transferred (2 × 106 or 4 × 104 cells) i.v. 1 day before infection.
Flow cytometry analysis
Gp33-H-2Db and np396-H-2Db tetramer production, surface and intracellular staining, and analysis by flow cytometry were performed as previously described.34 AV-phycoerythrin was obtained from Immunotools, Friesoythe, Germany, and 7-AAD from eBioscience. Anti-ceramide monoclonal antibodies (Enzo Life Sciences, Farmingdale, NY, USA) were used in combination with fluorescein isothiocyanate (FITC) Goat anti-mouse IgG (BD Pharmingen). Anti-CD3, anti-CD8, anti-IFN-γ, anti-IRF4, anti-CD45.1, and anti-CD45.2 were obtained from eBioscience. Intracellular staining with anti-Caspase 3, active form FITC (BD Pharmingen) was performed using a FoxP3 staining kit (eBioscience).
Secondary challenge with Vaccinia-gp
C57BL/6 mice were injected i.v. with 2 × 105 WT or Irf4−/− P14 T cells 1 day before i.v. infection with 2000 PFU LCMV-ARM. After 254 days (8.5 months), mice were infected with 2 × 106 PFU of a LCMV-GP recombinant Vaccinia virus (Vaccina-gp) (provided by D Bishop, Oxford University, Oxford, UK).24 Three days after infection with Vaccinia-gp, mice were killed and splenocytes were used for cytotoxicity assays.
Cytotoxic lymphocyte assay
Analysis of cytotoxic capacity of mixed splenocyte populations was conducted as previously described.34 Briefly, EL4 cells, pre-pulsed with gp33 or np396, or with MB6 (KAVVNIATM, P14-cell-specific35) peptides, and preincubated with Cr51, were mixed with splenocytes at indicated effector/target ratios. Cytotoxicity was determined by release of Cr51 into the cell culture supernatant after 6 h.
Immunoblotting and ELISA
Immunoblotting was performed as previously described31 using anti-IRF4 (Santa Cruz, Dallas, TX, USA), anti-BATF (Cell Signaling, Beverly, MA, USA), and anti-β-Actin antibodies (Cell Signaling). IL-2 enzyme-linked immunosorbent assay (ELISA) was performed using cell-free supernatants from conditioned T-cell medium and a mouse IL-2 ELISA Ready-SET-Go! Kit following the manufacturer’s instructions (eBioscience).
Statistical analysis
Data are expressed as mean±S.E.M. Statistical significance between two groups was analyzed using Student’s t-test. For experiments involving analysis of multiple groups, two-way analysis of variance with an additional Bonferroni post-test was used. The Mantel–Cox test was used for analysis of survival curves. P-values<0.05 were considered statistically significant.
Abbreviations
- 7-AAD:
-
7-aminoactinomycin D
- ALT:
-
alanine aminotransferase
- AP-1:
-
activator protein-1
- ARM:
-
LCMV Armstrong strain
- AV:
-
annexin V
- BATF:
-
B cell-activating transcription factor
- BM:
-
bone marrow
- CD:
-
cluster of differentiation
- CFSE:
-
carboxyfluorescein succinimidyl ester
- ELISA:
-
enzyme-linked immunosorbent assay
- FITC:
-
fluorescein isothiocyanate
- gp:
-
glycoprotein
- HCV:
-
hepatitis C virus
- HIV:
-
human immunodeficiency virus
- IFN-γ:
-
interferon-γ
- IL:
-
interleukin
- IL7R:
-
interleukin-7 receptor
- IRF4:
-
interferon regulatory factor 4
- KLRG1:
-
killer cell lectin-like receptor subfamily G member 1
- LCMV:
-
lymphocytic choriomeningitis virus
- LN:
-
lymph node
- mAb:
-
monoclonal antibody
- MFI:
-
mean fluorescence intensity
- np:
-
nucleoprotein
- OVA:
-
ovalbumin
- PE:
-
phycoerythrin
- PFU:
-
plaque-forming unit
- QVD:
-
Q-VD-OPh hydrate
- Rag1:
-
recombination activating gene 1
- SEM:
-
standard error of the mean
- TCR:
-
T-cell receptor
- tet:
-
tetramer
- Th:
-
T helper
- TNF:
-
tumor necrosis factor
- Treg:
-
regulatory T cell
- WT:
-
wild type
References
Rehermann B, Nascimbeni M . Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5: 215–229.
Walker BD, Yu XG . Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 2013; 13: 487–498.
Mittrucker HW, Matsuyama T, Grossman A, Kundig TM, Potter J, Shahinian A et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 1997; 275: 540–543.
Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci USA 2002; 99: 11808–11812.
Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH . Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med 2002; 195: 1003–1012.
Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 2009; 458: 351–356.
Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010; 33: 192–202.
Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol 2007; 8: 958–966.
Nayar R, Enos M, Prince A, Shin H, Hemmers S, Jiang JK et al. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc Natl Acad Sci USA 2012; 109: E2794–E2802.
Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest 2013; 123: 247–260.
Raczkowski F, Ritter J, Heesch K, Schumacher V, Guralnik A, Hocker L et al. The transcription factor interferon regulatory factor 4 is required for the generation of protective effector CD8+ T cells. Proc Natl Acad Sci USA 2013; 110: 15019–15024.
Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol 2013; 14: 1155–1165.
Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH et al. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity 2013; 39: 833–845.
Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 2012; 338: 975–980.
Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 2012; 490: 543–546.
Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F et al. A validated regulatory network for Th17 cell specification. Cell 2012; 151: 289–303.
Murphy TL, Tussiwand R, Murphy KM . Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat Rev Immunol 2013; 13: 499–509.
Kuroda S, Yamazaki M, Abe M, Sakimura K, Takayanagi H, Iwai Y . Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proc Natl Acad Sci USA 2011; 108: 14885–14889.
Zinkernagel RM, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A . T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med 1986; 164: 1075–1092.
Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 2008; 14: 756–761.
Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM . Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 1989; 342: 559–561.
Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE . RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992; 68: 869–877.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687.
Hany M, Oehen S, Schulz M, Hengartner H, Mackett M, Bishop DH et al. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol 1989; 19: 417–424.
Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature 2009; 460: 405–409.
McIlwain DR, Berger T, Mak TW . Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2013; 5: a008656.
He MX, He YW . A role for c-FLIP(L) in the regulation of apoptosis, autophagy, and necroptosis in T lymphocytes. Cell Death Differ 2013; 20: 188–197.
Tripathi P, Koss B, Opferman JT, Hildeman DA . Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ 2013; 20: 998–1007.
Wherry EJ . T cell exhaustion. Nat Immunol 2011; 12: 492–499.
Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 2010; 16: 1147–1151.
McIlwain DR, Lang PA, Maretzky T, Hamada K, Ohishi K, Maney SK et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science 2012; 335: 229–232.
Lang PA, Shaabani N, Borkens S, Honke N, Scheu S, Booth S et al. Reduced type I interferon production by dendritic cells and weakened antiviral immunity in patients with Wiskott-Aldrich syndrome protein deficiency. J Allergy Clin Immunol 2013; 131: 815–824.
Lang PA, Recher M, Honke N, Scheu S, Borkens S, Gailus N et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 2010; 52: 25–32.
Lang PA, Xu HC, Grusdat M, McIlwain DR, Pandyra AA, Harris IS et al. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ 2013; 20: 649–658.
Bachmann MF, Speiser DE, Ohashi PS . Functional management of an antiviral cytotoxic T-cell response. J Virol 1997; 71: 5764–5768.
Acknowledgements
We grateful for the technical assistance of Eugen Bäcker and Stefanie Münch. This study was supported by the Alexander von Humboldt Foundation (SKA2008 and SKA2010), the German Research Council (SFB974, LA2558/3-1, LA2558/4-1, LA2558/5-1, TRR60, LA1419/5-1, SCHE692/3-1, SCHE962/4-1), the Strategic Research Fund and VIVID graduate school of the Heinrich-Heine-University and the NIH Tetramer Facility. SQC is a Banting Fellow. ML was funded by the DZIF and the Behring-Röntgen-Stiftung. CRT is supported by the Swiss National Science Foundation (PBZHP3_141467).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by J Cidlowski
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Grusdat, M., McIlwain, D., Xu, H. et al. IRF4 and BATF are critical for CD8+ T-cell function following infection with LCMV. Cell Death Differ 21, 1050–1060 (2014). https://doi.org/10.1038/cdd.2014.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.19
Keywords
This article is cited by
-
BATF and BATF3 deficiency alters CD8+ effector/exhausted T cells balance in skin transplantation
Molecular Medicine (2024)
-
Effects of altered glycolysis levels on CD8+ T cell activation and function
Cell Death & Disease (2023)
-
RNA-sequencing data-driven dissection of human plasma cell differentiation reveals new potential transcription regulators
Leukemia (2021)
-
TIGIT limits immune pathology during viral infections
Nature Communications (2020)
-
BATF Interference Blocks Th17 Cell Differentiation and Inflammatory Response in Hepatitis B Virus Transgenic Mice
Digestive Diseases and Sciences (2019)