Abstract
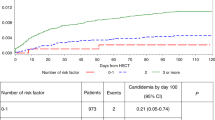
Patients with prior invasive fungal infection (IFI) increasingly proceed to allogeneic hematopoietic cell transplantation (HSCT). However, little is known about the impact of prior IFI on survival. Patients with pre-transplant IFI (cases; n=825) were compared with controls (n=10247). A subset analysis assessed outcomes in leukemia patients pre- and post 2001. Cases were older with lower performance status (KPS), more advanced disease, higher likelihood of AML and having received cord blood, reduced intensity conditioning, mold-active fungal prophylaxis and more recently transplanted. Aspergillus spp. and Candida spp. were the most commonly identified pathogens. 68% of patients had primarily pulmonary involvement. Univariate and multivariable analysis demonstrated inferior PFS and overall survival (OS) for cases. At 2 years, cases had higher mortality and shorter PFS with significant increases in non-relapse mortality (NRM) but no difference in relapse. One year probability of post-HSCT IFI was 24% (cases) and 17% (control, P<0.001). The predominant cause of death was underlying malignancy; infectious death was higher in cases (13% vs 9%). In the subset analysis, patients transplanted before 2001 had increased NRM with inferior OS and PFS compared with later cases. Pre-transplant IFI is associated with lower PFS and OS after allogeneic HSCT but significant survivorship was observed. Consequently, pre-transplant IFI should not be a contraindication to allogeneic HSCT in otherwise suitable candidates. Documented pre-transplant IFI is associated with lower PFS and OS after allogeneic HSCT. However, mortality post transplant is more influenced by advanced disease status than previous IFI. Pre-transplant IFI does not appear to be a contraindication to allogeneic HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the transplant associated infection surveillance network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–1100.
Barnes PD, Marr KA . Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol 2007; 139: 519–531.
Hayes-Lattin B, Maziarz RT . Update in the epidemiology, pophylaxis, and treatment of fungal infections in patients with hematologic disorders. Leuk Lymph 2004; 45: 669–680.
Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J . Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med 2007; 147: 400–411.
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood and Marrow Transplant Clinical Trials Network. Blood 2010; 116: 5111–5118.
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408–415.
Marr KA, Bow E, Chiller T, Maschmeyer G, Ribaud P, Segal B et al. Fungal infection prevention after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44: 483–487.
Hermann S, Klein SA, Jacobi V, Thalhammer A, Bialleck H, Duchscherer M et al. Older patients with high-risk fungal infections can be successfully allografted using non-myeloablative conditioning in combination with intensified supportive care regimens. Br J Haematol 2001; 113: 446–454.
Bachanova V, Brunstein CG, Burns LJ, Miller JS, Luo X, Defor T et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant 2009; 43: 237–244.
Aki ZS, Sucak GT, Yeğin ZA, Guzel O, Erbas G, Senol E . Hematopoietic stem cell transplantation in patients with active fungal infection: not a contraindication for transplantation. Transplant Proc 2008; 40: 1579–1585.
Fukuda T, Boeckh M, Guthrie KA, Mattson DK, Owens S, Wald A et al. Invasive aspergillosis before allogeneic hematopoietic stem cell transplantation: 10-year experience at a single transplant center. Biol Blood Marrow Transplant 2004; 10: 494–503.
Martino R, Parody R, Fukuda T, Maertens J, Theunissen K, Ho A et al. Impact of the intensity of the pretransplantation conditioning regimen in patients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: A retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 2006; 108: 2928–2936.
Penack O, Tridello G, Hoek J, Socie G, Blaise D, Passweg J et al. Influence of pre-existing invasive aspergillosis on allo-HSCT outcome: a retrospective EBMT analysis by the Infectious Diseases and Acute Leukemia Working Parties. Bone Marrow Transplantation 2016; 51: 418–423.
Vaidya SJ, Ortín M, López-Duarte M, Sirsohi B, Powles R, Treleaven J et al. Haemopoietic progenitor cell transplantation in patients with previous history of invasive fungal infection. Leuk Lymphoma 2005; 46: 1143–1150.
Girmenia C, Raiola AM, Piciocchi A, Stanzani M, Cudillo L, Pecoraro C et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 872–880.
Girmenia D, Barosi G, Piciocchi A, Arcese W, Aversa F, Bacigalupo A et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 1080–1088.
Andersen PK, Klein JP, Rosthøj S . Generalized linear models for correlated pseudo-observations with applications to multi-state models. Biometrika 2003; 90: 15–27.
Klein JP, Andersen PK . Regression modeling of competing risks data based on pseudo-values of the cumulative incidence function. Biometrics 2005; 61: 223–229.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347.
Hahn T, McCarthy PL Jr, Hassebroek A, Bredeson C, Gajewski J, Hale G et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol 2013; 31: 2437–2449.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363: 2091–2101 23.
Dvorak CC, Fisher BT, Sung L, Steinbach WJ, Nieder M, Alexander S et al. Antifungal prophylaxis in pediatric hematology/oncology: new choices & new data. Pediatr Blood Cancer 2012; 59: 21–26.
Norkin M, Wingard JR . Diagnostic strategies for invasive fungal infections in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. JNCCN 2013; 11: 941–949.
Cordeiro Rde A, Macedo Rde B, Teixeira CE, Marques FJ, Bandeira Tde J, Moreira JL et al. The calcineurin inhibitor cyclosporin A exhibits synergism with antifungals against Candida parapsilosis species complex. J Med Microbiol 2014; 63: 936–944.
Li H, Chen Z, Zhang C, Gao Y, Zhang X, Sun S . Resistance reversal induced by a combination of fluconazole and tacrolimus (FK506) in Candida glabrata. J Med Microbiol 2015; 64: 44–52.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer / Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/ MSG) consensus group. Clin Infect Dis 2008; 46: 1813–1821.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15: 1143–1238.
Young JA, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant 2016; 22: 359–370.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the US Government. *Corporate Members
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Maziarz, R., Brazauskas, R., Chen, M. et al. Pre-existing invasive fungal infection is not a contraindication for allogeneic HSCT for patients with hematologic malignancies: a CIBMTR study. Bone Marrow Transplant 52, 270–278 (2017). https://doi.org/10.1038/bmt.2016.259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.259
This article is cited by
-
Current Role of Allogeneic Stem Cell Transplantation in Multiple Myeloma
Oncology and Therapy (2022)
-
Allogeneic Hematopoietic Stem Cell Transplant in a Pediatric Patient with Invasive Fungal Infections: Challenges and Indications
Current Fungal Infection Reports (2021)