Abstract
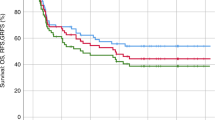
Reduced-intensity conditioning (RIC) has offered many primary immunodeficiency disorder (PID) patients who are ineligible for myeloablative regimens a chance of cure. However, the beneficial role of RIC was questioned following reports suggesting higher chance of rejection and lower symptom resolution rate in mixed chimerism settings. Forty-five children affected by PIDs with a median age of 21 months underwent allogeneic hematopoietic stem cell transplantation in our institute from 2007 to 2013. All patients received an identical RIC regimen. Forty-one patients had successful primary engraftment (91%). Of the successful engraftments, 80% (n=33) had stable full donor chimerism at last contact. Overall, eleven transplant-related mortalities were reported including five patients due to sepsis, three children due to grade IV acute GvHD, two due to chronic GvHD and one patient due to sepsis after primary graft failure. The median post-transplantation follow-up of deceased patients was 55 days. Five-year overall survival and disease-free survival was 75.6% and 68.89%, respectively. All surviving patients with successful engraftment became disease free, regardless of having full or mixed chimerism. Our study suggests that RIC regimen provides satisfactory rates of successful engraftment and full chimerism. Furthermore, patients with mixed chimerism were stable in long-term follow-up and this chimerism status offered the potential to resolve symptoms of immunodeficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, Cunningham-Rundles C et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2014; 5: 162.
Lee AJ, Wu J, Villegas MS, Shek LP, Lee BW, Tan PL . Stem cell transplantation for primary immunodeficiency disease: experience of a Singapore hospital. World Allergy Organ J 2012; 5: 41–44.
Hamidieh AA, Pourpak Z, Alimoghaddam K, Movahedi M, Bahoush G, Behmanesh F et al. Successful allogeneic stem cell transplantation with a reduced-intensity conditioning in a leukocyte adhesion deficiency type I patient. Pediatr Transplant 2011; 15: E30–E33.
Hamidieh AA, Pourpak Z, Hamdi A, Nabavi M, Ghavamzadeh A . Successful fludarabine-based hematopoietic stem cell transplantation in a pediatric patient with idiopathic CD4+ lymphocytopenia. Pediatr Transplant 2013; 17: E109–E111.
Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B et al. Bone marrow transplantation for severe combined immune deficiency. JAMA 2006; 295: 508–518.
Buckley RH . A historical review of bone marrow transplantation for immunodeficiencies. J Allergy Clin Immunol 2004; 113: 793–800.
Gennery AR, Cant AJ . Cord blood stem cell transplantation in primary immune deficiencies. Curr Opin Allergy Clin Immunol 2007; 7: 528–534.
Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D et al. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood 2005; 105: 879–885.
Giralt S . Reduced-intensity conditioning regimens for hematologic malignancies: what have we learned over the last 10 years? Hematology Am Soc Hematol Educ Program 2005; 2005: 384–389.
Satwani P, Cooper N, Rao K, Veys P, Amrolia P . Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant 2008; 41: 173–182.
Ringden O, Erkers T, Aschan J, Garming-Legert K, Le Blanc K, Hagglund H et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukaemia undergoing allogeneic haematopoietic stem cell transplantation. J Intern Med 2013; 274: 153–162.
Veys P . Reduced intensity transplantation for primary immunodeficiency disorders. Immunol Allergy Clin North Am 2010; 30: 103–124.
Chiesa R, Veys P . Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol 2012; 8: 255–266 quiz 67.
Amrolia P, Gaspar HB, Hassan A, Webb D, Jones A, Sturt N et al. Nonmyeloablative stem cell transplantation for congenital immunodeficiencies. Blood 2000; 96: 1239–1246.
Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood 2004; 104: 1550–1558.
Satwani P, Jin Z, Duffy D, Morris E, Bhatia M, Garvin JH et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant 2013; 19: 552–561.
Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant 2003; 9: 519–528.
Veys P, Rao K, Amrolia P . Stem cell transplantation for congenital immunodeficiencies using reduced-intensity conditioning. Bone Marrow Transplant 2005; 35: S45–S47.
Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA . Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968; 2: 1366–1369.
Bach FH, Albertini RJ, Joo P, Anderson JL, Bortin MM . Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet 1968; 2: 1364–1366.
Rondon G, Saliba RM, Khouri I, Giralt S, Chan K, Jabbour E et al. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transplant 2008; 14: 859–866.
Fleischhauer K, Locatelli F, Zecca M, Orofino MG, Giardini C, De Stefano P et al. Graft rejection after unrelated donor hematopoietic stem cell transplantation for thalassemia is associated with nonpermissive HLA-DPB1 disparity in host-versus-graft direction. Blood 2006; 107: 2984–2992.
Hamidieh AA, Pourpak Z, Hosseinzadeh M, Fazlollahi MR, Alimoghaddam K, Movahedi M et al. Reduced-intensity conditioning hematopoietic SCT for pediatric patients with LAD-1: clinical efficacy and importance of chimerism. Bone Marrow Transplant 2012; 47: 646–650.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sale GE, Lerner KG, Barker EA, Weiden PL, Sullivan K et al. Chronic cutaneous graft-versus-host disease in man. Am J Pathol 1978; 91: 545–570.
US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics: US Department of Health and Human Services Food and Drug Administration; 2007 [cited 2013 December 13]. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071590.pdf.
Hamidieh AA, Pourpak Z, Yari K, Fazlollahi MR, Hashemi S, Behfar M et al. Hematopoietic stem cell transplantation with a reduced-intensity conditioning regimen in pediatric patients with Griscelli syndrome type 2. Pediatr Transplant 2013; 17: 487–491.
Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ, Veys P et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol 2010; 126: 602–10 e1-e11.
Hamidieh AA, Pourpak Z, Hashemi S, Yari K, Fazlollahi MR, Movahedi M et al. Fludarabine-based reduced-intensity conditioning regimen for hematopoietic stem cell transplantation in primary hemophagocytic lymphohistiocytosis. Eur J Haematol 2014; 92: 331–336.
Burroughs L, Woolfrey A . Hematopoietic cell transplantation for treatment of primary immune deficiencies. Cell Ther Transplant 2010; 2.
Liesveld JL, Rothberg PG . Mixed chimerism in SCT: conflict or peaceful coexistence? Bone Marrow Transplant 2008; 42: 297–310.
Slatter MA, Rao K, Amrolia P, Flood T, Abinun M, Hambleton S et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood 2011; 117: 4367–4375.
Mitchell R, Nivison-Smith I, Anazodo A, Tiedemann K, Shaw P, Teague L et al. Outcomes of hematopoietic stem cell transplantation in primary immunodeficiency: a report from the Australian and New Zealand Children's Haematology Oncology Group and the Australasian Bone Marrow Transplant Recipient Registry. Biol Blood Marrow Transplant 2013; 19: 338–343.
Bojanic I, Golubic Cepulic B . [Umbilical cord blood as a source of stem cells]. Acta Med Croatica 2006; 60: 215–225.
Angarano R, Donnini I, Bartolozzi B, Bosi A . Double cord blood transplantation. Drugs Cell Ther Hematol 2013; 2: 113–121.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010; 11: 653–660.
Thomas C, Le Deist F, Cavazzana-Calvo M, Benkerrou M, Haddad E, Blanche S et al. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood 1995; 86: 1629–1635.
Al-wahadneh AM, Haddadin I, Hamouri M, Omari K, Aejellat F . Bone marrow transplantation for leukocyte adhesion deficiency-I: case report. Saudi J Kidney Dis Transpl 2006; 17: 564–567.
Morio T, Atsuta Y, Tomizawa D, Nagamura-Inoue T, Kato K, Ariga T et al. Outcome of unrelated umbilical cord blood transplantation in 88 patients with primary immunodeficiency in Japan. Br J Haematol 2011; 154: 363–372.
Triplett BM, Wang C, Yang J, Dallas M, Hartford C, Howard V et al. Effects of conditioning regimens and T cell depletion in hematopoietic cell transplantation for primary immune deficiency. Biol Blood Marrow Transplant 2012; 18: 1911–1920.
Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides [Internet]. 2012. Available from http://www.cibmtr.org.
Rousso SZ, Shamriz O, Zilkha A, Braun J, Averbuch D, Or R et al. Hematopoietic stem cell transplantations for primary immune deficiencies: 3 decades of experience from a Tertiary Medical Center. J Pediatr Hematol Oncol 2015; 37: e295–e300.
Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med 2014; 371: 434–446.
Ozyurek E, Cowan MJ, Koerper MA, Baxter-Lowe LA, Dvorak CC, Horn BN . Increasing mixed chimerism and the risk of graft loss in children undergoing allogeneic hematopoietic stem cell transplantation for non-malignant disorders. Bone Marrow Transplant 2008; 42: 83–91.
Hoelle W, Beck JF, Dueckers G, Kreyenberg H, Lang P, Gruhn B et al. Clinical relevance of serial quantitative analysis of hematopoietic chimerism after allogeneic stem cell transplantation in children for severe aplastic anemia. Bone Marrow Transplant 2004; 33: 219–223.
Willasch A, Hoelle W, Kreyenberg H, Niethammer D, Handgretinger R, Lang P et al. Outcome of allogeneic stem cell transplantation in children with non-malignant diseases. Haematologica 2006; 91: 788–794.
Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood 2011; 118: 1675–1684.
Slatter MA, Cant AJ . Hematopoietic stem cell transplantation for primary immunodeficiency diseases. Ann NY Acad Sci 2011; 1238: 122–131.
El-Cheikh J, Vazquez A, Crocchiolo R, Furst S, Calmels B, Castagna L et al. Acute GVHD is a strong predictor of full donor CD3+ T cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Am J Hematol 2012; 87: 1074–1078.
Acknowledgements
We are grateful for physicians’ help in diagnosing and referring patients. We sincerely thank all patients and their families for their participation in this study and the medical, nursing and clinical staff for their devoted care of patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hamidieh, A., Behfar, M., Pourpak, Z. et al. Long-term outcomes of fludarabine, melphalan and antithymocyte globulin as reduced-intensity conditioning regimen for allogeneic hematopoietic stem cell transplantation in children with primary immunodeficiency disorders: a prospective single center study. Bone Marrow Transplant 51, 219–226 (2016). https://doi.org/10.1038/bmt.2015.277
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.277
This article is cited by
-
A fludarabine and melphalan reduced-intensity conditioning regimen for HSCT in fifteen chronic granulomatous disease patients and a literature review
Annals of Hematology (2022)
-
Hematopoietic Cell Transplantation with Reduced Intensity Conditioning Using Fludarabine/Busulfan or Fludarabine/Melphalan for Primary Immunodeficiency Diseases
Journal of Clinical Immunology (2021)