Abstract
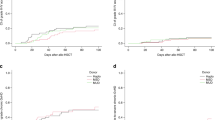
The ideal immunosuppressive agents to complement post-transplant cyclophosphamide (PTCy) in PBSC-based haploidentical hematopoietic cell transplantation (haplo-HCT) remain debated. This study looks at our experience with ATG-PTCy-Cyclosporine (CsA) prophylaxis in PB haplo-HCT since 2015. Between October 2015 and December 2021, 157 adults underwent haploidentical hematopoietic cell transplantation (haplo-HCT) using a GVHD prophylaxis regimen comprising rabbit-ATG, PTCy, and CsA. Among these patients, 76.4% received a total ATG dose of 4.5 mg/kg, and 23.5% received 2 mg/kg. T-cell replete peripheral blood stem cell (PBSC) grafts were infused on day 0. The study reported a median follow-up of 32 months (range 0.3–61.64) for survivors. The cumulative incidence of grade II–IV and grade III–IV acute GVHD at day +100 was 26.3% and 9.5%, respectively. Moderate/severe chronic GVHD at 1 year was 19.9%. The 2-year overall survival (OS) was 49.4%, with a relapse-free survival (RFS) of 44.6%. In multivariate analysis, older patients, and those with high/very-high disease risk indices (DRI) were at higher risk for worse OS and higher non-relapse mortality (NRM). The study confirms that using PTCy and ATG (4.5 mg/kg), alongside CsA is safe and effective in preventing GVHD when using peripheral blood as the stem cell source in haploidentical hematopoietic cell transplantation (haplo-HCT).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data are available upon request from the authors at their discretion.
References
Rimando J, McCurdy SR, Luznik L. How I prevent GVHD in high-risk patients: posttransplant cyclophosphamide and beyond. Blood. 2023;141:49–59.
Auletta JJ, Kou J, Chen M, Bolon YT, Broglie L, Bupp C, et al. Real-world data showing trends and outcomes by race and ethnicity in allogeneic hematopoietic cell transplantation: a report from the center for international blood and marrow transplant research. Transpl Cell Ther. 2023;29:346.e1–346.e10.
Fuchs EJ, McCurdy SR, Solomon SR, Wang T, Herr MR, Modi D, et al. HLA informs risk predictions after haploidentical stem cell transplantation with posttransplantation cyclophosphamide. Blood. 2022;139:1452–68.
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–61.
McCurdy SR, Radojcic V, Tsai HL, Vulic A, Thompson E, Ivcevic S, et al. Signatures of GVHD and relapse after posttransplant cyclophosphamide revealed by immune profiling and machine learning. Blood. 2022;139:608–23.
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex–incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64.
Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–204.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48.
Suárez EU. Hottest topics in hematopoietic stem cell transplantation: a summary from the 8th International Transplant and Cellular Therapy Course. Clin Hematol Int [Internet]. Oct 18 [cited 2023 Dec 13];5. Available from: https://chi.scholasticahq.com/article/89034-hottest-topics-in-hematopoietic-stem-cell-transplantation-a-summary-from-the-8th-international-transplant-and-cellular-therapy-course (2023).
Gooptu M, Romee R, St. Martin A, Arora M, Al Malki M, Antin JH, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138:273–82.
Barkhordar M, Kasaeian A, Janbabai G, Mousavi SA, Fumani HK, Tavakoli S, et al. Outcomes of haploidentical peripheral stem cell transplantation with combination of post-transplant cyclophosphamide (PTCy) and anti-thymocyte globulin (ATG) compared to unrelated donor transplantation in acute myeloid leukemia: A retrospective 10-year experience. Leuk Res. 2022;120:106918.
Saliba RM, Alousi AM, Pidala J, Arora M, Spellman SR, Hemmer MT, et al. Characteristics of graft-versus-host disease (GvHD) after post-transplantation cyclophosphamide versus conventional GvHD prophylaxis. Transpl Cell Ther. 2022;28:681–93.
Bejanyan N, Pidala JA, Wang X, Thapa R, Nishihori T, Elmariah H, et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv. 2021;5:1154–63.
Auletta J, Chen M, Shaw B. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, 2021. [Internet]. Power Point Presentation presented at; [cited 2023 Feb 28]. Available from: https://cibmtr.org/CIBMTR/Resources/Summary-Slides-Reports
Salas MQ, Charry P, Pedraza A, Martínez-Cibrian N, Solano MT, Domènech A, et al. PTCY and tacrolimus for GVHD prevention for older adults undergoing HLA-matched sibling and unrelated donor AlloHCT. Transpl Cell Ther. 2022;28:489.e1–489.e9.
Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant cyclophosphamide versus thymoglobulin in hla-mismatched unrelated donor transplant for acute myelogenous leukemia and myelodysplastic syndrome. Transpl Cell Ther. 2021;27:760–7.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Xu X, Yang J, Cai Y, Li S, Niu J, Zhou K, et al. Low dose anti-thymocyte globulin with low dose posttransplant cyclophosphamide (low dose ATG/PTCy) can reduce the risk of graft-versus-host disease as compared with standard-dose anti-thymocyte globulin in haploidentical peripheral hematopoietic stem cell transplantation combined with unrelated cord blood. Bone Marrow Transpl. 2021;56:705–8.
Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. N. Engl J Med. 2012;367:1487–96.
Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. Cham: Springer International Publishing [cited 2023 Mar 24]. Available from: http://link.springer.com/10.1007/978-3-030-02278-5; (2019)
Martino R, Romero P, Subirá M, Bellido M, Altés A, Sureda A, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. Bone Marrow Transpl. 1999;24:283–7.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Alfaro Moya T, Mattsson J, Remberger M, Lipton JH, Kim DD, Viswabandya A, et al. Influence of conditioning regimen intensity on outcomes post‐allogeneic hematopoietic cell transplantation for acute myeloid leukemia in complete morphological remission. Eur J Haematol. 2023;111:553–61.
Bashey A, Zhang MJ, McCurdy SR, St. Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Battipaglia G, Labopin M, Blaise D, Diez-Martin JL, Bazarbachi A, Vitek A, et al. Impact of the addition of antithymocyte globulin to post-transplantation cyclophosphamide in haploidentical transplantation with peripheral blood compared to post-transplantation cyclophosphamide alone in acute myelogenous leukemia: a retrospective study on behalf of the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Transpl Cell Ther. 2022;28:587.e1–587.e7.
Salas MQ, Atenafu EG, Law AD, Lam W, Pasic I, Chen C, et al. Experience using anti-thymocyte globulin with post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis in peripheral blood haploidentical stem cell transplantation. Transpl Cell Ther. 2021;27:428.e1–428.e9.
Moore J, Hamad N, Gottlieb D, Bajel A, Ritchie D, Yeung D, et al. Early cessation of calcineurin inhibitors is feasible post–haploidentical blood stem cell transplant: the ANZHIT 1 study. Blood Adv. 2023;7:5554–65.
Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, busulfan, and fludarabine conditioning regimen in T cell-replete HLA-haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2019;25:1407–15.
El-Cheikh J, Devillier R, Dulery R, Massoud R, Al Chami F, Ghaoui N, et al. Impact of adding antithymocyte globulin to posttransplantation cyclophosphamide in haploidentical stem-cell transplantation. Clin Lymphoma Myeloma Leuk. 2020;20:617–23.
Sun YQ, Wang Y, Wang FR, Yan CH, Cheng YF, Chen YH, et al. Graft failure in patients with hematological malignancies: a successful salvage with a second transplantation from a different haploidentical donor. Front Med. 2021;8:604085.
Giménez E, Torres I, Albert E, Piñana JL, Hernández-Boluda JC, Solano C, et al. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A systematic review, meta-analysis, and meta-regression analysis. Am J Transpl. 2019;19:2479–94.
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, Van Der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus–related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the infectious diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802.
Styczynski J, Reusser P, Einsele H, De La Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transpl. 2009;43:757–70.
Carreira AS, Salas MQ, Remberger M, Basso IN, Law AD, Lam W, et al. Bloodstream Infections and Outcomes Following Allogeneic Hematopoietic Cell Transplantation: A Single-Center Study. Transpl Cell Ther. 2022;28:50.e1–50.e8.
Duléry R, Malard F, Brissot E, Banet A, Sestili S, Belhocine R, et al. Reduced post-transplant cyclophosphamide dose with antithymocyte globulin in peripheral blood stem cell haploidentical transplantation. Bone Marrow Transplant [Internet]. Aug 18 [cited 2023 Oct 13]; Available from: https://www.nature.com/articles/s41409-023-02085-2 (2023).
Raiola AM, Angelucci E, Sica S, Bacigalupo A. Haploidentical bone marrow transplants with post transplant cyclophosphamide on day + 3 + 5: The Genova protocol. Blood Rev. 2023;62:101031.
Author information
Authors and Affiliations
Contributions
TA, MQS and AV designed the study. EA did the statistical analysis. TA, MQS, and AV interpreted the results, and wrote the manuscript. ASC, AL, WL, IP, DK, FM, AG, JL, RK and JM provided valuable input into the study and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alfaro Moya, T., Salas, M.Q., Santos Carreira, A. et al. Dual T cell depletion for graft versus host disease prevention in peripheral blood haploidentical hematopoietic cell transplantation for adults with hematological malignancies. Bone Marrow Transplant 59, 534–540 (2024). https://doi.org/10.1038/s41409-024-02216-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02216-3