Abstract
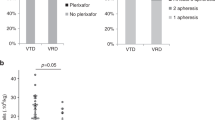
Initial therapy of multiple myeloma with lenalidomide-based regimens can compromise stem cell collection, which can be overcome with the addition of plerixafor. Plerixafor is typically given subcutaneously (SQ), with collection ∼11 h later for maximum yield. Intravenous administration may allow more rapid and predictable mobilization. This trial was designed to assess the efficacy and feasibility of IV plerixafor in patients receiving initial therapy with a lenalidomide-based regimen. Patients received G-CSF at 10 μg/kg/day for 4 days followed by IV plerixafor at 0.24 mg/kg/dose starting on day 5; plerixafor was administered early in the morning with apheresis 4–5 h later. Thirty-eight (97%) patients collected at least 3 × 106 CD34+ cells/kg within 2 days of apheresis. The median CD34+ cells/kg after 1 day of collection was 3.9 × 106 (range: 0.7–9.2) and after 2 days of collection was 6.99 × 106 (range: 1.1–16.5). There were no grade 3 or 4 non-hematological adverse events, and one patient experienced grade 4 thrombocytopenia. The most common adverse events were nausea, diarrhea and abdominal bloating. IV plerixafor is an effective strategy for mobilization with low failure rate and is well tolerated. It offers flexibility with a schedule of early-morning infusion followed by apheresis later in the day.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc 2009; 84: 1095–1110.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Kumar S . Multiple myeloma—current issues and controversies. Cancer Treat Rev 2010; 36 (Suppl 2): S3–S11.
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia 2009; 23: 1904–1912.
Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood 2009; 114: 1729–1735.
Sinha S, Gastineau D, Micallef I, Hogan W, Ansell S, Buadi F et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant 2011; 46: 943–949.
Knudsen LM, Rasmussen T, Jensen L, Johnsen HE . Reduced bone marrow stem cell pool and progenitor mobilisation in multiple myeloma after melphalan treatment. Med Oncol 1999; 16: 245–254.
Boccadoro M, Palumbo A, Bringhen S, Merletti F, Ciccone G, Richiardi L et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica 2002; 87: 846–850.
de la Rubia J, Blade J, Lahuerta JJ, Ribera JM, Martinez R, Alegre A et al. Effect of chemotherapy with alkylating agents on the yield of CD34+ cells in patients with multiple myeloma. Results of the Spanish Myeloma Group (GEM) Study. Haematologica 2006; 91: 621–627.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia 2008; 22: 1282–1284.
Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S . Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia 2008; 22: 1280–1281, author reply 1281-1282.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 718–723.
Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant 2009; 15: 249–256.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
Mohty M, Duarte RF, Croockewit S, Hubel K, Kvalheim G, Russell N . The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia 2011; 25: 1–6.
Micallef IN, Sinha S, Gastineau DA, Wolf R, Inwards DJ, Gertz MA et al. Cost-Effectiveness Analysis of a Risk-Adapted Algorithm of Plerixafor Use for Autologous Peripheral Blood Stem Cell Mobilization. Biol Blood Marrow Transplant 2013; 19: 87–93.
Nademanee AP, DiPersio JF, Maziarz RT, Stadtmauer EA, Micallef IN, Stiff PJ et al. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant 2012; 18: 1564–1572.
Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ et al. Effectiveness and cost analysis of "just-in-time" salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion 2011; 51: 2175–2182.
Abhyankar S, DeJarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J . A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant 2012; 47: 483–487.
Chen AI, Bains T, Murray S, Knight R, Shoop K, Bubalo J et al. Clinical experience with a simple algorithm for plerixafor utilization in autologous stem cell mobilization. Bone Marrow Transplant 2012; 47: 1526–1529.
Costa LJ, Abbas J, Hogan KR, Kramer C, McDonald K, Butcher CD et al. Growth factor plus preemptive ('just-in-time') plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone Marrow Transplant 2012; 47: 1403–1408.
Gopal AK, Karami M, Mayor J, Macebeo M, Linenberger M, Bensinger WI et al. The effective use of plerixafor as a real-time rescue strategy for patients poorly mobilizing autologous CD34(+) cells. J Clin Apher 2012; 27: 81–87.
Horwitz ME, Chute JP, Gasparetto C, Long GD, McDonald C, Morris A et al. Preemptive dosing of plerixafor given to poor stem cell mobilizers on day 5 of G-CSF administration. Bone Marrow Transplant 2012; 47: 1051–1055.
Cooper DL, Pratt K, Baker J, Medoff E, Conkling-Walsh A, Foss F et al. Late afternoon dosing of plerixafor for stem cell mobilization: a practical solution. Clin Lymphoma Myeloma Leuk 2011; 11: 267–272.
Burgstaler EA, Winters JL . Manual color monitoring to optimize hematopoietic progenitor cell collection on the Fenwal Amicus. J Clin Apher 2011; 26: 123–130.
Burgstaler EA, Pineda AA, Winters JL . Hematopoietic progenitor cell large volume leukapheresis (LVL) on the Fenwal Amicus blood separator. J Clin Apher 2004; 19: 103–111.
DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009; 27: 4767–4773.
Micallef IN, Ho AD, Klein LM, Marulkar S, Gandhi PJ, McSweeney PA . Plerixafor (Mozobil) for stem cell mobilization in patients with multiple myeloma previously treated with lenalidomide. Bone Marrow Transplant 2011; 46: 350–355.
Malard F, Kroger N, Gabriel IH, Hubel K, Apperley JF, Basak GW et al. Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide. Biol Blood Marrow Transplant 2012; 18: 314–317.
Acknowledgements
This work is supported in part by the Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA96028), Mayo Clinic Cancer Center and the Mayo Foundation. Clinical trial support is provided by Genzyme Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SKK has research support for clinical trials from Celgene, Millennium, Novartis and Genzyme and is a consultant for Merck. AD and MQL received clinical trial support from Celgene.
Additional information
Author contributions
SKK, BL and KL designed the study, collected and analyzed the data and wrote the manuscript; JM, MQL, FKB, DD, MAG, TM, MM, LB, SRHG, CR, AKS and AD contributed patients and were involved in writing the manuscript; DAG and JLW managed the apheresis unit and were involved in writing the manuscript.
Rights and permissions
About this article
Cite this article
Kumar, S., Mikhael, J., LaPlant, B. et al. Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy. Bone Marrow Transplant 49, 201–205 (2014). https://doi.org/10.1038/bmt.2013.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.175