Abstract
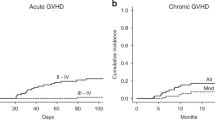
We previously reported that reduced intensity conditioning (RIC) regimen with fludarabine, BU and 2.5 mg/kg of rabbit anti-thymocyte globulin (r-ATG) was effective but associated with a high rate of acute and chronic GVHD. Therefore, we increased the dose of r-ATG to 5 mg/kg. In this report, we analyzed 87 patients with AML or myelodysplastic syndrome (MDS) undergoing allo-SCT from an HLA-identical sibling donor from 2000 to 2010. RIC consisted of fludarabine, BU and r-ATG 2.5 mg/kg on 1 day (r-ATG1; n=53) or 2.5 mg/kg per day over 2 days (r-ATG2; n=22). Grade 2–4 acute GVHD incidence at day 100 was 30.2% and 8.8% in the r-ATG1 and r-ATG2 groups, respectively (P=0.038). Extensive chronic GVHD incidence was 60.4% and 12% in the r-ATG1 and r-ATG2 groups, respectively (P<0.001). The relapse incidences (RI) at 24 months were 18.9% and 28.5% in r-ATG1 and r-ATG2 groups, respectively (P=0.640). Overall and PFS were not different between the r-ATG1 and r-ATG2 groups. r-ATG dose at 5 mg/kg in the setting of RIC seems a good balance allowing GVHD prevention and antitumor effect with a remarkable reduction of GVHD incidence without an identical level of increased relapse rate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Keating S, de WT, Suciu S, Willemze R, Hayat M, Labar B et al. The influence of HLA-matched sibling donor availability on treatment outcome for patients with AML: an analysis of the AML 8A study of the EORTC Leukaemia Cooperative Group and GIMEMA. European Organization for Research and Treatment of Cancer. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol 1998; 102: 1344–1353.
Zittoun RA, Mandelli F, Willemze R, de WT, Labar B, Resegotti L et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med 1995; 332: 217–223.
Castagna L, Furst S, Marchetti N, El CJ, Faucher C, Mohty M et al. Retrospective analysis of common scoring systems and outcome in patients older than 60 years treated with reduced-intensity conditioning regimen and alloSCT. Bone Marrow Transplant 2011; 46: 1000–1005.
McClune BL, Weisdorf DJ, Pedersen TL, Tunes da SG, Tallman MS, Sierra J et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol 2010; 28: 1878–1887.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 2009; 27: 4570–4577.
Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood 2004; 104: 1550–1558.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A . Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia 2010; 24: 1050–1052.
Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia 2005; 19: 916–920.
Blaise D, Vey N, Faucher C, Mohty M . Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2007; 92: 533–541.
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 2859–2867.
Sayer HG, Kroger M, Beyer J, Kiehl M, Klein SA, Schaefer-Eckart K et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant 2003; 31: 1089–1095.
Blaise D, Farnault L, Faucher C, Marchetti N, Furst S, El CJ et al. Reduced-intensity conditioning with Fludarabin, oral Busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol 2010; 38: 1241–1250.
Faucher C, Mohty M, Vey N, Gaugler B, Bilger K, Moziconnacci MJ et al. Bone marrow as stem cell source for allogeneic HLA-identical sibling transplantation following reduced-intensity preparative regimen. Exp Hematol 2003; 31: 873–880.
Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Blaise D, Bay JO, Faucher C, Michallet M, Boiron JM, Choufi B et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood 2004; 103: 435–441.
Blaise DP, Michel BJ, Faucher C, Mohty M, Bay JO, Bardoux VJ et al. Reduced intensity conditioning prior to allogeneic stem cell transplantation for patients with acute myeloblastic leukemia as a first-line treatment. Cancer 2005; 104: 1931–1938.
Mohty M, Boiron JM, Damaj G, Michallet AS, Bay JO, Faucher C et al. Graft-versus-myeloma effect following antithymocyte globulin-based reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant 2004; 34: 77–84.
Mohty M, de LH, El-Cheikh J, Ladaique P, Faucher C, Furst S et al. Reduced intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia: long term results of a ‘donor’ versus ‘no donor’ comparison. Leukemia 2009; 23: 194–196.
Hamadani M, Blum W, Phillips G, Elder P, Andritsos L, Hofmeister C et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2009; 15: 1422–1430.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Kroger N, Brand R, van BA, Zander A, Dierlamm J, Niederwieser D et al. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica 2009; 94: 542–549.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Amer Statist Assn 1958; 53: 457–481.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Cox DR . Regression models and life tables. Journal of the Royal Statistical Society: Serie B (Methodological) 1972; 34: 187–220.
Mohty M, Faucher C, Vey N, Stoppa AM, Viret F, Chabbert I et al. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant 2000; 26: 251–255.
Duggan P, Booth K, Chaudhry A, Stewart D, Ruether JD, Gluck S et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant 2002; 30: 681–686.
Bacigalupo A . Antithymocyte globulin for prevention of graft-versus-host disease. Curr Opin Hematol 2005; 12: 457–462.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009; 10: 855–864.
Mohty M . Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21: 1387–1394.
Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia 2011; 25: 932–938.
Mounier N, Haioun C, Cole BF, Gisselbrecht C, Sebban C, Morel P et al. Quality of life-adjusted survival analysis of high-dose therapy with autologous bone marrow transplantation versus sequential chemotherapy for patients with aggressive lymphoma in first complete remission. Groupe d’Etude les Lymphomes de l’Adulte (GELA). Blood 2000; 95: 3687–3692.
Lim Z, Brand R, Martino R, van BA, Finke J, Bacigalupo A et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol 2010; 28: 405–411.
Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem Cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant 2011; 17: 1490–1496.
Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 2003; 9: 320–329.
Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer 2009; 115: 1899–1905.
Cahu X, Mohty M, Faucher C, Chevalier P, Vey N, El-Cheikh J et al. Outcome after reduced-intensity conditioning allogeneic SCT for AML in first complete remission: comparison of two regimens. Bone Marrow Transplant 2008; 42: 689–691.
Acknowledgements
This work was supported by the following Grants: Pole ARECA from the Association pour la Recherche Contre le Cancer (ARC) (2001) and Programme Hospitalier de Recherche Clinique from ministry of health (2001). We are highly indebted to Pr William I Bensinger (FHCRC, Seattle, WA, USA) for his advices and critical review of this manuscript. We thank the nursing staff for providing excellent care for our patients. We also thank the following physicians at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care: A Charbonnier, E D’incan, J Rey, C Oudin and A Granata.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Pr Blaise has contributed to educational events sponsored by Genzyme. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Devillier, R., Crocchiolo, R., Castagna, L. et al. The increase from 2.5 to 5 mg/kg of rabbit anti-thymocyte-globulin dose in reduced intensity conditioning reduces acute and chronic GVHD for patients with myeloid malignancies undergoing allo-SCT. Bone Marrow Transplant 47, 639–645 (2012). https://doi.org/10.1038/bmt.2012.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.3
Keywords
This article is cited by
-
ATG or no ATG? – survey of clinical practice in EBMT centers on behalf of the Transplant Complications Working Party of EBMT
Bone Marrow Transplantation (2023)
-
Outcome of peripheral blood stem cell transplantation from HLA-identical sibling donors for adult patients with aplastic anemia
International Journal of Hematology (2023)
-
Comparison of myeloablative and reduced intensity conditioning unrelated donor allogeneic peripheral blood stem cell transplant outcomes for AML using thymoglobulin for GVHD prophylaxis
Annals of Hematology (2021)
-
Impact of anti-thymocyte globulin dose for graft-versus-host disease prophylaxis in allogeneic hematopoietic cell transplantation from matched unrelated donors: a multicenter experience
Annals of Hematology (2021)
-
Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel
Bone Marrow Transplantation (2020)