Abstract
Background:
Epithelial cell adhesion molecule is overexpressed in bladder tumours and released from bladder cancer cells in vitro. We test the hypotheses that urinary EpCAM could act as a biomarker for primary bladder cancer detection and risk stratification.
Methods:
Epithelial cell adhesion molecule was measured by ELISA in urine from 607 patients with primary bladder tumours and in urine from 53 non-cancer controls. Mann–Whitney tests and ROC analyses were used to determine statistical significance and discrimination between non-cancer controls and different stages and grades of disease. Multivariable modelling and Kaplan–Meier analyses were used to determine prognostic significance. The structure of urinary EpCAM was investigated by western blotting and mass spectrometry.
Results:
Urinary EpCAM levels increase with stage and grade of bladder cancer. Alongside grade and stage, elevated urinary EpCAM is an independent indicator of poor prognosis with a hazard ratio of 1.76 for bladder cancer-specific mortality. The soluble form of EpCAM in urine is the extracellular domain generated by cleavage between ala243 and gly244. Further studies are required to define the influence of other urinary tract malignancies and benign urological conditions on urinary EpCAM.
Conclusion:
The extracellular domain of EpCAM is shed into urine by bladder tumours. Urinary EpCAM is a strong indicator of bladder cancer-specific survival, and may be useful within a multi-marker panel for disease detection or as a stand-alone marker to prioritise the investigation and treatment of patients. The mechanisms and effects of EpCAM cleavage in bladder cancer are worthy of further investigation, and may identify novel therapeutic targets.
Similar content being viewed by others
Main
Urothelial bladder cancer (UBC) is the fifth most common cancer in Western societies accounting for 38 200 and 17 000 deaths per year in the EU and USA, respectively, and with a rising global incidence (Ploeg et al, 2009; Burger et al, 2013). The majority of patients present with painless visible haematuria, and at presentation over 75% of UBCs detected by cystoscopy are non-muscle-invasive tumours (NMIBC: stages Ta/T1/Tcis; Burger et al, 2013). For these patients, long-term cystoscopic surveillance is required following an initial endoscopic tumour resection (Babjuk et al, 2011). Such surveillance is invasive, uncomfortable, time consuming and expensive (Mowatt et al, 2010). Muscle-invasive disease (MIBC: stages T2+) requires more radical treatment and carries a 5-year survival rate of only 27–50% (Stenzl et al, 2011). Consequently, an important goal in UBC research is the development of non-invasive tests to reduce reliance upon cystoscopy for detection and surveillance. Commercially available urinary biomarkers are either soluble urinary proteins (e.g., NMP22, BTA) or based on the detection of tumour cells in urine (ImmunoCyt, UroVysion; reviewed in Tilki et al (2011)). Some of the protein-based tests have higher sensitivity for early disease than cytology, but lower specificity (Hennenlotter et al, 2011; Miyake et al, 2012a, 2012b). Recently, nucleic acid markers (e.g., DNA methylation, mutations, microRNAs) have shown promise for UBC detection (Reinert et al, 2011; Miah et al, 2012; Allory et al, 2013; Zuiverloon et al, 2013).
In addition, whereas clinical and pathological factors enable risk stratification, molecular markers could potentially provide additional prognostic information to further inform management where decision-making can be difficult, such as in the treatment of highest risk NMIBC (Babjuk et al, 2011), or the utilisation of bladder-preserving strategies for MIBC (James et al, 2012). To our knowledge no individual urinary protein biomarker has shown an independent prognostic value, although urine protein-based tests are desirable due to the ease of measurement and the potential for point of care testing.
We recently published an analysis of the proteins secreted by UBC cell lines (Shimwell et al, 2013), and now report a large-scale evaluation of one of these proteins, EpCAM, as a urinary biomarker for the detection and risk stratification of UBC. As EpCAM is an integral membrane protein and not expected to be present in urine, we also investigated whether EpCAM is released intact or by shedding of the extracellular domain.
Patients and Methods
Patient samples
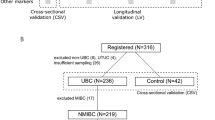
Urine samples were prospectively collected for biomarker research between 2006 and 2009 as part of the Bladder Cancer Prognosis Programme (ethics approval 06/MRE04/65; Zeegers et al, 2010). Patients were enrolled into the study on the basis of initial cystoscopic findings suggestive of primary UBC. All patients were newly diagnosed with UBC, and had not received treatment for UBC prior to urine collection. Subsequently, all patients were treated according to current standard practice. Inclusion and exclusion criteria are described in detail elsewhere (Zeegers et al, 2010). Mid-stream urine was collected prior to endoscopic tumour resection (TURBT). Samples were placed on ice, centrifuged at 2000 r.p.m. for 10 min within 8 h of collection and the supernatants stored at −80 °C. As patient recruitment occurred prior to histopathological confirmation of UBC, a proportion of patients were ultimately diagnosed with non-malignant conditions and these serve as the non-cancer ‘controls’. Patient information is shown in Table 1.
ELISAs
Urine samples were vortexed at room temperature and centrifuged at 15 000 g for 5 min prior to use. The EpCAM ELISA utilises capture and detection antibodies raised against the extracellular domain of recombinant human EpCAM (DY960; R&D Systems Europe Ltd, Abingdon, UK). To each well of the assay were added 100 μl of urine and 50 μl of PBS+1% BSA, and seven-point calibration curves constructed using two-fold dilutions of 1 ng ml−1 standard.
Data analysis
EpCAM levels are presented as medians and statistical significance was calculated using Mann–Whitney tests. Bladder cancer-specific survival was defined as the time from registration into the BCPP study to date of death from bladder cancer. Patients were censored at the date last known to be alive or date of non-bladder cancer-related death. Univariable Cox proportional hazards models were employed (alpha 0.1) to identify factors to be included in a multivariable model; significance was set at 0.05. Analysis was done in Stata 12.1 (StataCorp, College Station, TX, USA).
Western blotting
Samples were boiled in SDS-PAGE sample loading buffer containing 50 mM DTT, separated with 4–12% NuPAGE gels, blotted onto PVDF and probed with biotinylated anti-EpCAM antibody (BAF960; R&D Systems Europe Ltd) and streptavidin-linked HRP. Selected samples were deglycosylated by incubating at 37 °C for 2 h with 100 units per ml PNGase F (F8435; Sigma-Aldrich Company Ltd., Dorset, UK) in 150 mM NaCl, 50 mM Tris (pH 7.4), 1% Triton X-100 and 50 mM DTT.
Affinity purification and mass spectrometry
Ten ml of urine was incubated with 5 μg of biotinlyated anti-EpCAM antibody and 100 μl of strepdavidin-coated beads at 4 °C overnight. The beads were washed with 150 mM NaCl, 50 mM Tris (pH 7.4), 0.05% Tween 20 and proteins eluted by boiling in SDS-PAGE sample loading buffer. Following SDS-PAGE, gel slices were destained, reduced with 50 mM DTT, alkylated with 100 mM iodoacetamide and digested overnight with 250 ng of sequencing grade trypsin or asp-N (Promega UK, Southampton, Hampshire, UK). Mass spectrometry details are provided in Supplementary Information.
Results
Urinary EpCAM levels in UBC
Compared to non-cancer controls, EpCAM was significantly elevated in patients with UBC (median 6.74 pg per mg creatinine vs 3.86, P=0.0025); however, the area under the ROC curve was only 0.625 and it was evident that elevation was confined to late stage and high-grade disease (Figures 1A–C). Stratifying by stage revealed significant elevation in T1 and T2+, but not Ta UBCs (P-values <0.0001, <0.0001 and 0.96, respectively). Stratifying by grade revealed no EpCAM elevation in G1 or G2 UBCs (P>0.05), but significant elevation in G3 UBCs compared to both control subjects and patients with G1 or G2 UBCs (P<0.0001).
Urinary EpCAM as a diagnostic marker
Using a threshold of 24 pg EpCAM per mg creatinine (mean±2 s.d. in the 53 control subjects), 5%, 20% and 38% of Ta, T1 and T2+ UBCs and 2%, 7% and 30% of G1, G2 and G3 UBCs, respectively, gave a positive test result with a false-positive rate of 6% in the controls. Thus, elevated urinary levels of EpCAM are highly indicative of the presence of T2+ or G3 UBC. The area under the ROC curve for detecting T2+ UBC was 0.805 (0.742–0.858, 95% CI), 0.765 for G3 UBC (0.716–0.809) and 0.725 for G3 NMIBC (0.659–0.784) (Figure 2A–C). At a specificity of 90% (79.3–96.8), the sensitivity for detecting MIBC was 59.6% (51.0–67.7), increasing to 72.3% (64.2–79.5) at 80% (68.0–90.5) specificity.
Detection of UBC using urinary EpCAM.The ROC curves show discrimination between stages of UBC and non-cancer controls (A) grades of UBC and non-cancer controls (B) and grades of NMIBC and non-cancer controls (C). Panel A: dotted line=Ta, dashed line=T1, solid line=T2+. Panels B and C: dotted line=grade 1, dashed line=grade 2, solid line=grade 3.
Urinary EpCAM as a prognostic marker
Univariable analyses included age (years), multiple tumours (1 vs 2+), grade (1, 2 vs 3), size of largest tumour (⩽3 cm vs >3 cm), CIS (present vs absent), stage (Ta or T1 vs T2+), sex (male vs female) and urinary EpCAM (normal vs elevated). Grade, stage, age, tumour size, CIS and EpCAM were statistically significant (P<0.005). Multivariable analysis showed that elevated urinary EpCAM (>24 pg EpCAM per mg creatinine) was an independent prognostic factor with a hazard ratio of 1.8 (95% CI 1.1, 2.7: P=0.012) for UBC-specific survival (Table 2 and Figure 3).
Urinary EpCAM characterisation
To determine whether urinary EpCAM is the intact membrane-bound protein or a soluble fragment, we measured EpCAM in six samples pre- and post-ultracentrifugation (140 000 g, 30 min): there was no difference in urinary EpCAM concentration, indicating that urinary EpCAM is not membrane bound. We then investigated EpCAM by western blotting, initially analysing UBC cell lines to determine the mobility of full-length EpCAM— this migrated as a single band at 48 kDa (Figure 4A). Urine from six UBC patients (>300 pg per ml−1 EpCAM) showed three bands with molecular weights of 42, 35 and 27 kDa, which varied in intensity between the patients (Figure 4B). Deglycosylation decreased the intensities of the higher molecular weight bands and increased those of the lower molecular weight bands (Figure 4C), indicating that the multiple bands are different glycoforms rather than different fragments of EpCAM. The molecular weight of the lowest band closely matched that calculated for the extracellular region of EpCAM (27.4 kDa). Mass spectrometry of tryptic digests of affinity purified urinary EpCAM identified peptides covering most of the extracellular domain and none from the transmembrane or intracellular domains (Figure 5 and Supplementary Information). The data revealed that the cleavage site resulting in shedding of the extracellular domain was either between lys242 and ala243 or between ala243 and gly244, immediately adjacent to the predicted transmembrane span. Using asp-N (instead of trypsin) we identified two further peptides (DEKAPEFSMQGLKA and DEKAPEFSMQGLK), unequivocally demonstrating that the cleavage site is ala243/gly244 with additional, possibly secondary, cleavage occurring at lys242/ala243.
Peptides identified by LC-MS/MS following trypsin digestion of immunoprecipitated EpCAM.Solid lines indicate peptides identified from urinary EpCAM. A peptide that could only be identified after PNGase treatment is indicated by the double-dashed line. Arrows indicate N-linked glycosylation motifs. An intracellular peptide that was only found in cell line EpCAM is indicated by the dotted line.
Urinary EpCAM stability
We incubated eight urine samples at 25 °C for up to 1 week. Four of these samples had high natural levels of EpCAM (>300 pg ml−1) and >90% of the EpCAM remained after 3 days (Figure 6). To test whether low levels of EpCAM in some urine samples might be due to faster degradation, we selected four samples with low levels of EpCAM (<50 pg ml−1) and added 200 ng ml−1 recombinant human EpCAM (the standard for the ELISA); in these samples degradation was slightly faster, with levels decreasing to 60% after 3 days.
EpCAM stability in urine.Urine samples were incubated for up to 1 week at 25 °C and EpCAM levels measured by ELISA. The data shown are the mean (s.e.m.) values from four urine samples with high levels of EpCAM (476–931 pg ml−1; open symbols) and four urine samples with low levels of EpCAM augmented with 200 pg ml−1 recombinant EpCAM.
Urinary EpCAM and haematuria
Haematuria did not significantly influence urinary EpCAM in the non-UBC or T2+ patient groups, although an association between haematuria and EpCAM was observed in NMIBCs (Table 3). However, there was no direct association between haematuria and urinary EpCAM; a strong correlation between urinary EpCAM and albumin concentration would be expected if blood or plasma leakage was responsible for elevating urinary EpCAM, but this was not the case (Supplementary Data).
Discussion
Elevated urinary EpCAM is observed in many patients with grade three and stage T2+ UBCs, and is a significant independent prognostic factor for UBC-specific survival. The predominant form of EpCAM in urine is not the intact protein, but a soluble and stable form comprised of the entire extracellular domain.
Urinary EpCAM appears to be specific to high-grade and stage UBC, as elevated levels were seen in only 2% of patients with grade one UBC, 7% with grade two but 30% with grade three, and only 5% of patients with Ta, 20% with T1 but 38% with T2+ UBC. Moreover, elevated urinary EpCAM increases the risk of UBC-specific death 1.8-fold. Thus, although sensitivity as a diagnostic marker for UBC is poor (and a role in surveillance unlikely), urinary EpCAM may have a role in rapidly detecting those patients with the most advanced and/or aggressive disease so that their investigation and management can be tailored and expedited accordingly. Although other urinary protein markers are also elevated in advanced UBC, their value as independent prognostic factors has not been demonstrated.
EpCAM is a type-1 cell surface protein comprised of 291 residues with the N-terminal 242 residues being extracellular, 23 residues predicted to form a transmembrane helix and the C-terminal 26 residues being located intracellularly (Szala et al, 1990). The extracellular part of the protein is glycosylated at asparagines 51, 88 and 175, and the region spanning residues 4—112 is stabilised by six intramolecular disulphide bonds (Chong and Speicher, 2001). The latter region has sequence similarity with the EGF and thyrobulin type-1 domain, whereas the C-terminal half of the extracellular region is unique and remains uncharacterised (Chong and Speicher, 2001).
EpCAM is overexpressed in many epithelial malignancies, including bladder CIS (Patriarca et al, 2009), and high-grade and advanced stage UBCs (Brunner et al, 2008). The tumour-specific expression of EpCAM has led to its use for capturing circulating UBC cells (Okegawa et al, 2010), and also as a target for directing therapies to bladder tumours (Kowalski et al, 2010). Serum levels of EpCAM have been measured previously – they are typically in the low ng ml−1 range (Abe et al, 2002; Petsch et al, 2011) and are slightly elevated in patients with oesophageal cancers (Kimura et al, 2007). Epithelial cell adhesion molecule expression is a prognostic indicator in several cancers; in UBC, Brunner et al (2008) reported that high tissue levels of EpCAM are associated with poor prognosis. Their study investigated 99 patients with various stages of UBC and looked at overall survival: multivariate analysis highlighted age, stage and number of recurrences, but not EpCAM expression, as independent factors. Urinary EpCAM concentration is likely to be dependent not only on tissue expression levels, but also on the rate of shedding into the urine. Our analysis of over 600 patients indicates that urinary EpCAM is a prognostic factor for UBC-specific survival, independent of grade and stage.
Despite its long association with cancer, the role of EpCAM has remained elusive with both tumour suppressor and oncogenic properties reported. Maetzel et al (2009) demonstrated that EpCAM can be sequentially cleaved to release its extracellular and intracellular domains, ‘EpEX’ and ‘EpICD’, respectively; EpICD diffuses into the nucleus and activates oncogenic signalling events (Maetzel et al, 2009; Chaves-Pérez et al, 2013). Our data indicate that these phenomena also occur in UBC, with urinary ‘EpEX’ associated with high-grade and stage UBCs with poor prognosis. In support of this, Ralhan et al (2010) recently performed immunohistochemistry for EpICD in a range of human epithelial cancers, including 10 cases of UBC, nine of which were positive for EpICD. In UBC we have shown that the extracellular domain of EpCAM is released by cleavage immediately adjacent to the cell membrane. The precise location of the cleavage that released EpEX was not described by Maetzel et al (2009), but the protease involved (TACE or ADAM 17) usually cleaves membrane proteins 10–15 residues away from the membrane surface (Coglievina et al, 2013), suggesting atypical cleavage or an alternative mechanism of extracellular domain release in UBC.
It is probable that a diagnostic test for UBC will require multiple markers to reach the high sensitivity and specificity required in the clinic. Elevated urinary EpCAM levels are highly indicative of MIBC, thus representing a candidate that could be incorporated into a test to stratify patients at presentation into those at low or high risk of harbouring MIBC, and subsequently tailoring their investigation and management (Shimwell et al, 2013). For example, staging CT or MRI for MIBC is typically performed after TURBT which can cause artefact, hindering local staging, misinforming clinical decisions and delaying definitive treatment. Urinary biomarkers could be used to expedite CT or MRI before TURBT, thus improving diagnosis and local staging, and potentially reducing delays. However, the main clinical benefit of urinary EpCAM may be its independent prognostic value, thus informing clinical decisions in a number of settings, such as in the treatment of highest risk NMIBC (Babjuk et al, 2011), or the utilisation of bladder-preserving strategies for MIBC (James et al, 2012).
A major strength of our approach is the prospective nature of the biospecimen collection, specifically undertaken to carry out such biomarker research (Zeegers et al, 2010). However, further studies in an independent cohort will be needed to validate our findings; such a cohort should include more non-UBC samples to thoroughly define the effects of benign bladder conditions and other urological malignancies on urinary EpCAM, and this work is ongoing. Although beyond the scope of this initial urinary EpCAM description and characterisation, future work should also investigate whether urinary EpCAM is a prognostic biomarker in recurrent as well as primary UBCs and whether it is predictive of therapeutic responses.
Conclusions
Urinary EpCAM may prove useful for facile identification of patients with high-risk poor-prognosis UBC. The overexpression of EpCAM in UBC (Brunner et al, 2008), its detection in conditioned media from the UBC cell lines (Shimwell et al, 2013) and the data presented here indicate that the source of the EpCAM extracellular domain in the urine of UBC patients is shedding directly from tumour cells. Further investigation of EpEX and EpICD in MIBC may reveal novel therapeutic targets.
Change history
04 February 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abe H, Kuroki M, Imakiire T, Yamauchi Y, Yamada H, Arakawa F, Kuroki M (2002) Preparation of recombinant MK-1/Ep-CAM and establishment of an ELISA system for determining soluble MK-1/Ep-CAM levels in sera of cancer patients. J Immunol Methods 270: 227–233.
Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, van der Keur K, Dyrskjot L, Lurkin I, Vermeij M, Carrato A, Lloreta J, Lorente J, Carrillo-de Santa Pau E, Masius R, Kogevinas M, Steyerberg E, van Tilborg A, Abas C, Orntoft T, Zuiverloon T, Malats N, Zwarthoff E, Real F (2013) Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 110 (3): 668–678.
Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, Rouprêt M European Association of Urology (2011) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59: 997–1008.
Brunner A, Prelog M, Verdorfer I, Tzankov A, Mikuz G, Ensinger C (2008) EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J Clin Pathol 61: 307–310.
Burger M, Catto J, Dalbagni G, Grossman H, Herr H, Karakiewicz P, Kassouf W, Kiemeney L, La Vecchia C, Shariat S, Lotan Y (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63: 234–241.
Chaves-Pérez A, Mack B, Maetzel D, Kremling H, Eggert C, Harréus U, Gires O (2013) EpCAM regulates cell cycle progression via control of cyclin D1 expression. Oncogene 32: 641–650.
Chong J, Speicher D (2001) Determination of disulfide bond assignments and N-glycosylation sites of the human gastrointestinal carcinoma antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J Biol Chem 276: 5804–5813.
Coglievina M, Guarnaccia C, Zlatev V, Pongor S, Pintar A (2013) Jagged-1 juxtamembrane region: biochemical characterization and cleavage by ADAM17 (TACE) catalytic domain. Biochem Biophys Res Commun 432: 666–671.
Hennenlotter J, Huber S, Todenhöfer T, Kuehs U, Schilling D, Aufderklamm S, Gakis G, Schwentner C, Stenzl A (2011) Point-of-care tests for bladder cancer: the influencing role of hematuria. Adv Urol 2011:, doi:10.1155/2011/937561.
James N, Hussain S, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart R Investigators B (2012) Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366: 1477–1488.
Kimura H, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, Miyazaki T, Masuda N, Fukuchi M, Kuwano H (2007) Prognostic significance of EpCAM expression in human esophageal cancer. Int J Oncol 30: 171–179.
Kowalski M, Entwistle J, Cizeau J, Niforos D, Loewen S, Chapman W, MacDonald G (2010) A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of non muscle-invasive bladder cancer in BCG refractory and BCG-intolerant patients. Drug Des Devel Ther 15: 313–320.
Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle P, Munz M, Gires O (2009) Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 11: 162–171.
Miah S, Dudziec E, Drayton R, Zlotta A, Morgan S, Rosario D, Hamdy F, Catto J (2012) An evaluation of urinary microRNA reveals a high sensitivity for bladder cancer. Br J Cancer 107: 123–128.
Miyake M, Goodison S, Giacoia E, Rizwani W, Ross S, Rosser C (2012a) Influencing factors on the NMP-22 urine assay: an experimental model. BMC Urol 12:, doi:10.1186/1471-2490-12-23.
Miyake M, Goodison S, Rizwani W, Ross S, Grossman H, Rosser C (2012b) Urinary BTA: indicator of bladder cancer or of hematuria. World J Urol 30: 869–873.
Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths T, N'Dow J, Nabi G, Cook J, Vale L (2010) Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess 14: 1–331.
Okegawa T, Hayashi K, Hara H, Nutahara K, Higashihara E (2010) Immunomagnetic quantification of circulating tumor cells in patients with urothelial cancer. Int J Urol 17: 254–258.
Patriarca C, Colombo P, Pio Taronna A, Wesseling J, Franchi G, Guddo F, Naspro R, Macchi R, Giunta P, Di Pasquale M, Parente M, Arizzi C, Roncalli M, Campo B (2009) Cell discohesion and multifocality of carcinoma in situ of the bladder: new insight from the adhesion molecule profile (e-cadherin, Ep-CAM, and MUC1). Int J Surg Pathol 17: 99–106.
Petsch S, Gires O, Rüttinger D, Denzel S, Lippold S, Baeuerle P, Wolf A (2011) Concentrations of EpCAM ectodomain as found in sera of cancer patients do not significantly impact redirected lysis and T-cell activation by EpCAM/CD3-bispecific BiTE antibody MT110. MAbs 3: 31–37.
Ploeg M, Aben K, Kiemeney L (2009) The present and future burden of urinary bladder cancer in the world. World J Urol 27: 289–293.
Ralhan R, He H, So A, Tripathi S, Kumar M, Hasan M, Kaur J, Kashat L, MacMillan C, Chauhan S, Freeman J, Walfish P (2010) Nuclear and cytoplasmic accumulation of Ep-ICD is frequently detected in human epithelial cancers. PLoS One 5: e14130.
Reinert T, Modin C, Castano F, Lamy P, Wojdacz T, Hansen L, Wiuf C, Borre M, Dyrskjøt L, Orntoft T (2011) Comprehensive genome methylation analysis in bladder cancer: identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin Cancer Res 17: 5582–5592.
Shimwell N, Bryan R, Wei W, James N, Cheng K, Zeegers M, Johnson P, Martin A, Ward D (2013) Combined proteome and transcriptome analyses for the discovery of urinary biomarkers for urothelial carcinoma. Br J Cancer 108: 1854–1861.
Stenzl A, Cowan N, De Santis M, Kuczyk M, Merseburger A, Ribal M, Sherif A, Witjes J (2011) Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 59: 1009–1018.
Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, Linnenbach A (1990) Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci USA 87: 3542–3546.
Tilki D, Burger M, Dalbagni G, Grossman H, Hakenberg O, Palou J, Reich O, Rouprêt M, Shariat S, Zlotta A (2011) Urine markers for detection and surveillance of non-muscle-invasive bladder cancer. Eur Urol 60: 484–492.
Zeegers M, Bryan R, Langford C, Billingham L, Murray P, Deshmukh N, Hussain S, James N, Wallace D, Cheng K (2010) The West Midlands Bladder Cancer Prognosis Programme: rationale and design. BJU Int 105: 784–788.
Zuiverloon T, Beukers W, van der Keur K, Nieuweboer A, Reinert T, Dyrskjot L, Orntoft T, Zwarthoff E (2013) Combinations of urinary biomarkers for surveillance of patients with incident nonmuscle invasive bladder cancer: the European FP7 UROMOL project. J Urol 189: 1945–1951.
Acknowledgements
We thank all the West Midlands Consultant Urologists and their units who are involved with BCPP, as well as the BCPP research nurses, and MR Grant, D Bird, J Barnwell, D Nekeman and EH van Roekel for contributing to these studies and for recruiting patients. BCPP is funded by Cancer Research UK, the University of Birmingham and the Birmingham & The Black Country, West Midlands North and South Comprehensive Local Research Networks, and sponsored by the University of Birmingham. The BCPP biospecimen collection was supported by funding from the Birmingham Experimental Cancer Medicine Centre. DG Ward was funded by the Birmingham Science City.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Bryan, R., Shimwell, N., Wei, W. et al. Urinary EpCAM in urothelial bladder cancer patients: characterisation and evaluation of biomarker potential. Br J Cancer 110, 679–685 (2014). https://doi.org/10.1038/bjc.2013.744
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.744
Keywords
This article is cited by
-
EpCAM tumor specificity and proteoform patterns in urothelial cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Antibody–Drug Conjugates in Uro-Oncology
Targeted Oncology (2022)
-
The Vitamin D status is associated with serum C-reactive protein and adhesion molecules in patients with renal cell carcinoma
Scientific Reports (2019)
-
Nanotechnology and cancer: improving real-time monitoring and staging of bladder cancer with multimodal mesoporous silica nanoparticles
Cancer Nanotechnology (2016)
-
Establishing guidelines for CAR-T cells: challenges and considerations
Science China Life Sciences (2016)