Abstract
Background:
Primary Ewing’s sarcoma family of tumours (ESFTs) may respond to chemotherapy, although many patients experience subsequent disease recurrence and relapse. The survival of ESFT cells following chemotherapy has been attributed to the development of resistant disease, possibly through the expression of ABC transporter proteins.
Methods:
MRP-1 and Pgp mRNA and protein expression in primary ESFTs was determined by quantitative reverse-transcriptase PCR (RT-qPCR) and immunohistochemistry, respectively, and alternative splicing of MRP-1 by RT-PCR.
Results:
We observed MRP-1 protein expression in 92% (43 out of 47) of primary ESFTs, and cell membrane MRP-1 was highly predictive of both overall survival (P<0.0001) and event-free survival (P<0.0001). Alternative splicing of MRP-1 was detected in primary ESFTs, although the pattern of splicing variants was not predictive of patient outcome, with the exception of loss of exon 9 in six patients, which predicted relapse (P=0.041). Pgp protein was detected in 6% (38 out of 44) of primary ESFTs and was not associated with patient survival.
Conclusion:
For the first time we have established that cell membrane expression of MRP-1 or loss of exon 9 is predictive of outcome but not the number of splicing events or expression of Pgp, and both may be valuable factors for the stratification of patients for more intensive therapy.
Similar content being viewed by others
Main
Current response rates of Ewing’s sarcoma family of tumour (ESFT) patients to first-line chemotherapy are as high as 77% (Bacci et al, 2000; Yonemori et al, 2007; Gaspar et al, 2012; Serlo et al, 2012), demonstrating that some ESFTs are chemoresponsive. However, others are resistant to chemotherapy at diagnosis (23%), and this is known as intrinsic resistance. Despite the high response rate, 20–40% of patients experience disease recurrence (Ahrens et al, 1999; Cotterill et al, 2000; Rodriguez-Galindo et al, 2007; Stahl et al, 2011) and only 10–23% are alive at 5 years (Barker et al, 2005; Huang and Lucas, 2011; Stahl et al, 2011). The survival of drug-resistant clones after initial chemotherapy and the high occurrence of relapse have been attributed to the development of acquired drug resistance. The ability to evade cell death in this way represents a major challenge for improving patient outcome and survival.
Several mechanisms have been implicated in the development of resistance, including the efflux of agents (Seidel et al, 1995; Panyam and Labhasetwar, 2003) by multidrug resistance (MDR) ABC transporter proteins (Oda et al, 1997), in particular the overexpression of Pgp and MRP-1. Increased expression of these efflux proteins results in the removal of chemotherapeutic agents from the cell, reduced effectiveness of chemotherapeutics and decreased survival (Burger et al, 2003). To date, Pgp is the only MDR protein to have been investigated in ESFTs. However, the results of published studies are contradictory, one suggesting that Pgp was not predictive of prognosis (Perri et al, 2001) and a second significantly linking expression of Pgp to poor response to chemotherapy (Roessner et al, 1993).
Therefore, in this study we have investigated the hypothesis that intrinsic drug resistance in primary ESFTs is mediated through the expression of the ABC transporter proteins Pgp and MRP-1, and that these may be predictive of patient outcome and response to therapy. As functional splice variants of MRP-1 have been described in some normal and cancer cells (Cole et al, 1992; Grant et al, 1997; He et al, 2004), we have also examined for the first time whether this arises in primary ESFTs, and whether the pattern of spliced variants might be prognostic.
Materials and methods
Patients and clinical data
Forty-nine cases with a confirmed diagnosis of primary ESFTs, made by independent pathology review and by reverse-transcriptase PCR (RT-PCR) detection of the EWS-ETS gene rearrangement or CD99 positive by immunohistochemistry (IHC), were included in the study (Table 3). Clinical data were provided by the Children’s Cancer and Leukaemia Group Data Centre, University of Leicester. Information was sought on the diagnosis date, date of relapse or death and current disease status. Patient age at diagnosis ranged from 2 to 38 years, with a median age of 13 years, and patients were 22 men and 25 women. Tumours were collected from 1993 to 2006, mounted in optimum cutting temperature (OCT) and stored in liquid nitrogen. Informed consent and ethical approval was obtained for the use of frozen tumour material in these biological studies (MREC 98/4/023, dated 24 July 2001).
Control cell lines and tissues
The substrate-adherent Ewing’s sarcoma TTC 466 and the neuroblastoma (NB) SK-N-SH cell lines were obtained and cultured as previously described (Roundhill and Burchill, 2012). Both cell lines are yeast-, bacterial- and mycoplasma-free and are determined every 4 months using the EZ-PCR mycoplasma test kit (Geneflow, Lichfield, UK). The breast adenocarcinoma was obtained from a local anonymised pathology tissue archive (LREC approval, dated 17 July 2001).
Immunohistochemistry for MRP-1 and Pgp in primary ESFTs
Immunohistochemistry for MRP-1 expression was performed on 47 tumours (Dalal et al, 2005). Sections (5 μm), fixed using methanol:acetone (1 : 1; Merck Biosciences, San Diego, CA, USA) for 2 × 2 min at room temperature on glass slides, were incubated with a polyclonal MRP-1 antibody (20 μg ml−1; A23, Axxora, Exeter, UK) and diluted in antibody diluent (DakoCytomation, Invitrogen, Paisley, UK) for 1 h, followed by an incubation with a secondary antibody (1 μg ml−1, goat anti-rabbit; DakoCytomation; diluted in antibody diluent) for 30 min, both at room temperature. Breast adenocarcinoma was used as a positive control for MRP-1 expression; optimum antibody concentration was determined empirically.
Pgp expression was detected in acetone-fixed (4 °C for 10 min) sections (5 μm) by using the EnVision+ System-HRP (DAB) kit (DakoCytomation). Sections were incubated with Pgp antibody (10 μg μl−1; JSB-1, Axxora) and were diluted in antibody diluent overnight at 4 °C. The SK-N-SH NB cell sections (5 μm), mounted in OCT compound, were used as a positive control for Pgp expression; optimum antibody concentration was determined empirically.
All sections were visualised using the Liquid DAB Substrate Chromogen System for peroxidase (DakoCytomation) and were counterstained with haematoxylin. Sections were visualised by light microscopy (Zeiss Axioplan microscope; Zeiss, Cambridge, UK). MRP-1 and Pgp expression were scored manually by two independent reviewers, using light microscopy to visualise the sections; each reviewer was blind to the clinical outcome data. MRP-1 expression was scored as the number of positively stained cells out of 100 in 4 randomly selected areas of each tumour; the number of cells with membrane localisation was also scored. Cells were scored as positive or negative for Pgp.
Extraction of DNA and RNA
Frozen primary ESFT were stained with haematoxylin, ESFT cells captured by laser capture microdissection (LCM). RNA and DNA from the cell lines were extracted as previously described, and quality and quantity were examined (Brownhill et al, 2007).
Quantitative RT-PCR for MRP-1 and Pgp
For the analysis of ABC transporter mRNA expression by quantitative RT-PCR (qRT-PCR), 10 ng or 1 ng per 1 μg of cell line RNA was amplified for MRP-1 and Pgp, respectively; 5 ng of RNA from LCM primary tumour was amplified for MRP-1 and Pgp. Total RNA was reverse transcribed using Superscript III Reverse Transcriptase (Invitrogen; Roundhill and Burchill, 2012). Samples were analysed in triplicate and in the absence of RT enzyme or without sample RNA (replaced with DNase-/RNase-free H2O; Invitrogen), to control for non-specific amplification of DNA and contamination, respectively.
Complementary DNA was added to a PCR mix containing sequence-specific reverse and forward primers (Supplementary Figure 1) and 1 × TaqMan Universal PCR Master Mix (Invitrogen). Samples were denatured for 2 min at 50 °C, Taq polymerase was activated by heating for 10 min at 95 °C and cDNA amplified using 40 cycles of 95 °C for 15 s and 60 °C for 1 min. mRNA expression was calculated using the comparative Ct method (Brownhill et al, 2007), relative to the housekeeping genes β-2-microglobulin (Brownhill et al, 2007) and peptidylprolyl isomerase A (PPIA) (Fischer et al, 2005; Lastowska et al, 2007), in cell lines (Supplementary Figure 2) and primary ESFTs, respectively. TTC 466 and SK-N-SH cell line RNA was included as a reference control for interassay variability in the amplification of MRP-1 and Pgp, respectively.
Identification of MRP-1 pre-RNA splicing
Total RNA (500 ng or 10 ng from cell lines (Supplementary Figure 2) and primary ESFTs, respectively) was reverse transcribed as previously described (Roundhill and Burchill, 2012) and cDNA added to a PCR mix containing MRP-1 exon-specific primers (Supplementary Figure 1) and 1 unit of AmpliTaq Gold DNA polymerase in 1 × AmpliTaq Reaction Buffer II (Invitrogen). The AmpliTaq gold was activated by one cycle at 95 °C for 10 min and cDNA amplified for 35 cycles of 95 °C for 30 s, 60 °C for 30 s and extension at 72 °C for 1 min. This was followed by a further final extension at 72 °C for 10 min. PCR products were separated by 2% agarose gel electrophoresis and were visualised after staining with ethidium bromide (0.5 μg ml−1; Sigma-Aldrich Company Ltd., Dorset, UK), under UV light.
Statistical analysis
The prognostic value of MRP-1 and Pgp expression, and one or more MRP-1 splicing events, was evaluated using the Cox proportional hazards model; results with P-values <0.05 were considered significant. Overall survival (OS) was calculated as the time from diagnosis to the date last seen, regardless of the number of events that may have occurred. Event-free survival was defined as the time from diagnosis to the time of first event; the first event could be relapse, death or date last seen for surviving patients. Patients without time to a first event or OS follow-up data were excluded from analyses; clinical follow-up information was available in 33 out of 38, 39 out of 44 and 44 out of 47 patients for alternative splicing, qRT-PCR and IHC, respectively. There was limited tumour tissue available from some patients and, hence, it was not possible to analyse all tumours for alternative splicing, protein and mRNA. The statistical methodology used to identify the optimal cut-point to maximise the difference in OS and time to a first event between patient groups with high and low MRP-1 expression is as previously described (London et al, 2005). Briefly, each decile of MRP-1 expression was evaluated for a statistically significant difference in OS and time to a first event. Specific associations between RNA and protein levels, and between individual splicing events, were determined by Spearmann’s rank and Pearson’s correlations, respectively.
Results
MRP-1 is more frequently expressed in primary ESFTs than in Pgp; the level of cell membrane MRP-1 expression predicts patient outcome
MRP-1 was expressed in 91% (43 out of 47) of the primary ESFTs analysed (Figures 1A and B); staining was absent in 4 out of 47 ESFTs (Figure 1C). The pattern of MRP-1 expression was similar across each tumour sample. Samples either expressed intracellular MRP-1 only (which was cytoplasmic and/or nuclear, 5 out of 43; Figure 1A), or both intracellular and membrane-localised MRP-1 (38 out of 43; Figure 1B). No tumour expressed MRP-1 in the membrane alone. MRP-1 total protein (MRP-1T) or membrane protein (MRP-1Mem) expression was not predictive of response to therapy measured by necrosis (MRP-1T, P=0.34; MRP-1Mem, P=0.09). However, MRP-1Mem but not MRP-1T was predictive of time to a first event (MRP-1T, P=0.096; MRP-1Mem, P<0.0001; cut-off upper 10% high expressers; Figure 1D) and OS (MRP-1T, P=0.75; MRP-1Mem, P<0.0001; cut-off upper 10% high expressers; Figure 1E) in these patients.
MRP-1 protein expression in primary ESFTs. Positive MRP-1 protein expression was (A) intracellular or (B) localised to the membrane and intracellular (n=45). (C) Some primary ESFT expressed no MRP-1 (n=4). N=nuclear; M=cell membrane MRP-1 staining. The relationship between cell membrane MRP-1 and (D) time to a first event or (E) OS was analysed by the Cox proportional hazards model (n=44). Grey dotted line=10% of tumours expressing MRP-1; blue solid line=remaining patients (lower 90% of tumours expressing MRP-1); n=number of tumours analysed.
All mRNAs from primary ESFTs (44) successfully amplified for the endogenous control gene PPIA (mean Ct=25, range=21–30), confirming the quality of the tumour RNA. Expression of MRP-1 mRNA was heterogeneous in primary ESFTs and was detected in 82% (36 out of 44) of tumours (Figure 2A; mean Ct=33, range 30–39). MRP-1 mRNA was detected in three out of four MRP-1-negative tumours. The level of MRP-1 mRNA was not predictive of response to therapy measured by necrosis (P=0.82) but was a predictor of time to a first event (P=0.03, 0.15; cut-off upper 20% and 10% high expressers, respectively; Figure 2B), but not OS (P=0.1; cut-off upper 10% high expressers; Figure 2C). There was no significant correlation between MRP-1 mRNA and MRP-1T (r=0.11) or MRP-1Mem (r=0.06) expression.
MRP-1 mRNA expression in primary ESFTs. (A) There was heterogeneous mRNA expression of MRP-1 in primary ESFT. MRP-1 mRNA expression was calculated using the comparative Ct method, relative to the housekeeping gene PPIA and the reference sample, the ESFT cell line TTC 466 (n=46); data presented as ΔΔCt. The relationship between mRNA expression and (B) time to a first event or (C) OS was analysed by the Cox proportional hazards model (n=39). Grey dotted line=10% of tumours expressing MRP-1; blue solid line=remaining patients (lower 90% of tumours expressing MRP-1); n=number of tumours analysed.
Pgp protein expression was identified in only 6% (3 out of 47) of the primary ESFTs analysed (Figures 3A and B), where expression was observed in the cell membrane and cytoplasm. Pgp was not expressed in the nucleus. Pgp protein expression was not predictive of patient response to treatment measured by necrosis (P=0.64), time to a first event (P=0.60) or OS (P=0.28). In contrast, Pgp mRNA was detected in 86% (38 out of 44) of tumour samples analysed (Figure 3C; mean Ct=34, range=30–39). The level of Pgp mRNA was not predictive of response to therapy measured by necrosis (P=0.22), time to a first event (P=0.25; cut-off upper 10% high expressers) or OS (P=0.20; cut-off upper 10% high expressers). Similar to MRP-1, there was no correlation between Pgp mRNA and protein expression (P=0.81).
Pgp expression in primary ESFTs. (A) Positive Pgp expression was observed in the cell membrane and cytoplasm of cells (n=3 tumours). (B) The majority of primary ESFTs were negative for Pgp expression (n=46). C=cytoplasmic and M=cell membrane Pgp staining. (C) There was heterogeneous mRNA expression of Pgp in primary ESFT. Pgp mRNA expression was calculated using the comparative Ct method, relative to the housekeeping gene PPIA and the reference sample, the NB cell line SK-N-SH; data presented as ΔΔCt; n=number of tumours analysed.
A cohort of primary ESFTs were analysed for both MRP-1 and Pgp, mRNA and protein. There was no correlation between the Pgp protein expression and membrane (P=0.97) or total (P=0.64) MRP-1 expression. However, there was a significant correlation between the levels of Pgp and MRP-1 mRNA (P=0.026).
MRP-1 pre-mRNA is alternatively spliced in primary ESFTs
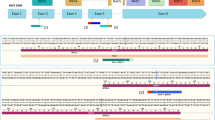
Alternative splicing of MRP-1 was observed in 63% (24 out of 38) of tumours (Figure 4 and Table 1). In all MRP-1 protein-positive samples, the full-length PCR product was amplified (Figure 4 and Table 1). The statistically significant paired exon losses (e.g., exon 5 and 6 together) are summarised in Table 2.Table 3
MRP-1 splicing events observed in ESFT primary tissues. Splicing events observed in primary ESFTs (sample 40) generated by RT-PCR, employing 11 primer mixes spanning the 31 exons of MRP-1 (Supplementary Figure 1). Image shows ethidium bromide-stained amplified products after separation by electrophoresis and visualisation under UV light. *=splice variant; →=full-length/predicted PCR product; ladder=50 bp DNA ladder;+=RT-positive amplified sample; – =RT-negative control; +H2O=control for contamination containing RT, but in which RNA is replaced with H2O; H2O=negative control in which cDNA is replaced with H2O in PCR step.
There is no correlation between the number of splicing events and the MRP-1 mRNA by RT-PCR (r2=0.1378, P=0.4159) and MRP-1MEM expression (r2=0.3070, P=0.0646) in primary ESFTs. However, MRP-1MEM and mRNA expression were significantly associated with loss of exons 5 or 6 (r2=0.3440, P=0.0371) and exon 17 (r2=0.3551, P=0.0336), respectively, suggesting that in conditions of high MRP-1 expression pre-RNA splicing may increase.
Although the loss of exons 6, 9, 23 and 29, which was frequently observed in this sample group, resulted in a frame shift (Table 1), which we anticipate would produce a non-functional protein, there is no correlation between time to a first event, OS and the number of or a particular splicing event (P>0.05). However, interestingly, patient relapse was significantly associated with loss of exon 9 (P=0.041) (Table 2).
Discussion
Cell membrane expression of MRP-1 in ESFTs at diagnosis strongly predicts for time to a first event and OS, consistent with the hypothesis that cell membrane MRP-1 is in part responsible for drug resistance in ESFT. This is the first study of total and membrane MRP-1 expression and prognostic value in primary ESFTs. MRP-1 protein was expressed in the nucleus and cell membrane of primary ESFTs, consistent with reports in other cancer types (Izquierdo et al, 1995; Maraldi et al, 1999; Molinari et al, 2002). Total MRP-1 expression did not correlate with time to first event, OS or necrosis, which is in agreement with previous studies evaluating total MRP-1 expression in paediatric and adult cancers (Izquierdo et al, 1995; Filipits et al, 1997; Styczynski et al, 2007). Similar to the current study, MRP-1 mRNA expression has been associated with time to a first event and OS in NB (Norris et al, 1996; Haber et al, 2006) and a panel of other childhood malignancies (Plasschaert et al, 2005; Schaich et al, 2005). However, in adult cancers, such as breast and ovarian carcinomas, MRP-1 mRNA expression was not predictive of time to a first event (Izquierdo et al, 1995; Burger et al, 2003), OS (Plasschaert et al, 2005) or necrosis (Burger et al, 2003), suggesting that MRP-1 may be more important in cancers of young people than in adult disease. Whether this reflects the developmental origin of the cancers requires further investigation.
Pgp was expressed in only 3 put of 47 primary ESFTs; whether this reflects methylation of the MDR1 gene as in ESFT cell lines (Supplementary Figure 2) remains to be seen. In agreement with the current work, Perri et al (2001) did not observe a correlation between Pgp protein expression and time to a first event (P=0.87) or OS (P=0.79) in patients at diagnosis. However, protein expression of Pgp has been associated with poor response to therapy (P<0.08; Roessner et al, 1993) in pre- and post-diagnosis ESFTs. Unfortunately, in this study the authors failed to distinguish between pre- and post-treatment groups, which might explain the difference in study conclusions. Increased Pgp protein expression has however been linked to an adverse OS in other paediatric (Baldini et al, 1995; Filipits et al, 1997) and adult cancers (Mignogna et al, 2006). We have also shown that Pgp mRNA expression was not predictive of patient outcome, in agreement with previous studies in children with NB (Norris et al, 1996; Haber et al, 1997). In contrast, Pgp mRNA expression in some paediatric and adult cancers taken at diagnosis has been associated with a worse OS (Schaich et al, 2005), time to a first event (Burger et al, 2003; Kourti et al, 2007) and response to treatment (Burger et al, 2003). As Pgp protein was expressed in only 3 out of 47 tumours, we agree it is difficult to conclude on the prognostic value of Pgp in the current study cohort. What we can conclude is that MRP-1 is the dominant predictive MDR protein in ESFTs taken at diagnosis. Whether Pgp is of additional predictive value at diagnosis in a small group of patients or has a role in drug-induced resistance remains to be seen.
Although significant correlations between the mRNA and protein expression of MDR proteins have been described (Nooter et al, 1995; Cai et al, 1999; Yang et al, 2002; Lacueva et al, 2005; Haber et al, 2006), in the current study there was no correlation between Pgp protein and mRNA, or full and membrane MRP-1 protein and mRNA in primary ESFTs. These MDR proteins are highly posttranscriptionally regulated by processes such as methylation (Kantharidis et al, 1997; Ando et al, 2000; Qiu et al, 2007). This is supported by the regulation of Pgp expression in cell lines by methylation of MDR1 (Supplementary Figure 2). No correlation was observed between total MRP-1 and Pgp protein expression, consistent with post-transcriptional regulation of the two proteins (Hipfner et al, 1997; Zhou, 2008). In agreement with previous studies (Almquist et al, 1995; Nooter et al, 1995; Lu et al, 2004), we observed a significant correlation between MRP-1 and Pgp mRNA, possibly highlighting a similar mechanism of transcriptional regulation. Interestingly, decreased expression of miR-326 and miR-451 has been associated with increased MRP-1 and Pgp expression, respectively, regulating gene transcription through the 3′-UTR of the ABC transporter proteins (Kutanzi et al, 2011). In addition, the chromosome rearrangement EWS-FLI1 (Burchill, 2003) has been shown to increase EYA3 through miR-708 (Robin et al, 2012). We are currently investigating the role of micro-RNAs in the development of MDR.
In this study, we have described for the first time the presence of MRP-1 splice variants in primary ESFTs. We found loss of exon 9 was predictive of patient relapse (five out of six patients remained in continuous complete remission) and so represents a prognostic marker that requires further investigation. Although splicing of MRP-1 has not previously been correlated with patient outcome, alternative splicing of P73 has been identified as a negative prognostic marker in patients with NB (Romani et al, 2003). In contrast, upregulation of the alternative MDM4 and MDM2 splice variants, described in soft tissue sarcomas (Bartel et al, 2005) and breast cancer patients (Lukas et al, 2001), have both been correlated with poor OS (Lukas et al, 2001; Bartel et al, 2005). Alternative splicing of exons 10–19 and exons 5, 13, 17, 18 and 30 in addition to WT MRP-1 expression, has previously been described in a range of cell types (Grant et al, 1997) and ovarian cancers (He et al, 2004), respectively. We also identified pre-RNA splicing of MRP-1 in both cancer and normal cell lines (Supplementary Figure 2), suggesting that splicing of MRP-1 is not a cancer-specific phenomenon. Interestingly, the splicing of 10 specific exons occurred at a significantly higher frequency in cell lines (Supplementary Figure 2) than in primary ESFTs, most likely reflecting changes in MRP-1 splicing following the adaptation of cells in vitro. Loss of exons 3, 5, 17 and 18 result in the loss of whole amino acids and loss of exons 6, 9, 23 and 29 each result in a frame shift, all of which have been observed in this study and are likely to produce a non-functional protein. In agreement with this, MRP-1 splice variants missing each of exon groups 12–26, 17 and 18, 5 and 30 were reported to all confer drug resistance in HEK293T cells when overexpressed, although the effect was not as significant as that of the full-length MRP-1 (He et al, 2004).
Although in this study we have not investigated the regulation of alternative splicing, overexpression of the splicing factors PTB and SRp20 (He et al, 2004) have been linked to increased MRP-1 splicing. Furthermore, the level of trans-acting and cis-elements, and the effects of external stimuli, have been reported to determine the accuracy of pre-mRNA splicing (Maniatis and Tasic, 2002), although the mechanism controlling these are not yet understood. Although some studies have suggested that EWS-ETS fusion proteins (Burchill, 2003) inhibit splicing of pre-RNA (Yang et al, 2000a, 2000b), EWS-FLI1 specifically has been shown to directly interact with the splicing factors U1C and SF1 (Knoop and Baker, 2001), and has been reported to increase transcript levels of cyclinD1b in ESFT cells through alternative splicing (Sanchez et al, 2008). Whether this is the case for MRP-1 in ESFT cells requires further investigation.
In addition to full-length MRP-1 protein, we have also identified novel shortened MRP-1 protein products (MRP-1-100); products of 65–190 kDa have been reported in a range of other cancer types (Krishnamachary et al, 1994; Regina et al, 1998; Nuti et al, 2000; Fernetti et al, 2001). As we have observed splicing of the MRP-1 pre-RNA in ESFT cell lines, it is possible that MRP-1-100 represents a protein product of alternative splicing, although this requires further investigation. Interestingly, alternatively spliced pre-RNA transcripts have been described in other members of the ABC transporter family, such as Pgp (Devine et al, 1991), BCRP (Nakanishi et al, 2006), ABCG1 (Engel et al, 2006) and ABCA1 (Bocchi et al, 2010), and have been associated with a change in protein activity, suggesting that abnormal splicing may represent an additional mechanism of ABC transporter expression control.
Although a variety of other mechanisms of MDR have been described in ESFTs, including defective DNA repair (Alldinger et al, 2007), overexpression of anti-apoptotic proteins (Batra et al, 2003), glutathione metabolism (Scotlandi et al, 2009) and the presence of cancer-initiating cells (Suva et al, 2009), the results of this study suggest that targeting the ABC transporter protein MRP-1 may be important to improve the response to therapy and survival for relapsed ESFT patients. High MRP-1 cell membrane protein expression might be useful to identify those patients that are most likely to benefit from more aggressive treatment, combination treatment with standard chemotherapeutics and MRP-1 inhibitors, or treatment with a non-MRP-1 substrate (Roundhill and Burchill, 2012). Similarly, patients with low MRP-1 or loss of MRP-1 exon 9 could be spared unnecessary toxicity, while decreasing the cost of treatment. Before any clinical benefit can be realised, it is now important to validate cell membrane MRP-1 and loss of exon 9 as a predictive marker in ESFTs, and to determine the independent clinical significance of these findings.
Change history
09 July 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ahrens S, Hoffmann C, Jabar S, Braun-Munzinger G, Paulussen M, Dunst J, Rube C, Winkelmann W, Heinecke A, Gobel U, Winkler K, Harms D, Treuner J, Jurgens H (1999) Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Cooperative Ewing Sarcoma Study. Med Pediatr Oncol 32: 186–195.
Alldinger I, Schaefer KL, Goedde D, Ottaviano L, Dirksen U, Ranft A, Juergens H, Gabbert HE, Knoefel WT, Poremba C (2007) Microsatellite instability in Ewing tumor is not associated with loss of mismatch repair protein expression. J Cancer Res Clin Oncol 133: 749–759.
Almquist KC, Loe DW, Hipfner DR, Mackie JE, Cole SP, Deeley RG (1995) Characterization of the M(r) 190,000 multidrug resistance protein (MRP) in drug-selected and transfected human tumor cell. Cancer Res 55: 102–110.
Ando T, Nishimura M, Oka Y (2000) Decitabine (5-Aza-2'-deoxycytidine) decreased DNA methylation and expression of MDR-1 gene in K562/ADM cells. Leukemia 14: 1915–1920.
Bacci G, Ferrari S, Bertoni F, Rimondini S, Longhi A, Bacchini P, Forni C, Manfrini M, Donati D, Picci P (2000) Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 18: 4–11.
Baldini N, Scotlandi K, Barbanti-Brodano G, Manara MC, Maurici D, Bacci G, Bertoni F, Picci P, Sottili S, Campanacci M, Serra M. (1995) Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N Engl J Med 333: 1380–1385.
Barker LM, Pendergrass TW, Sanders JE, Hawkins DS (2005) Survival after recurrence of Ewing’s sarcoma family of tumors. J Clin Oncol 23: 4354–4362.
Bartel F, Schulz J, Bohnke A, Blumke K, Kappler M, Bache M, Schmidt H, Wurl P, Taubert H, Hauptmann S (2005) Significance of HDMX-S (or MDM4) mRNA splice variant overexpression and HDMX gene amplification on primary soft tissue sarcoma prognosis. Int J Cancer 117: 469–475.
Batra S, Perelman N, Luck LR, Shimada H, Malik P (2003) Pediatric tumor cells express erythropoietin and a functional erythropoietin receptor that promotes angiogenesis and tumor cell survival. Lab Invest 83: 1477–1487.
Bocchi L, Pisciotta L, Fasano T, Candini C, Puntoni MR, Sampietro T, Bertolini S, Calandra S (2010) Multiple abnormally spliced ABCA1 mRNAs caused by a novel splice site mutation of ABCA1 gene in a patient with Tangier disease. Clin Chim Acta 411: 524–530.
Brownhill SC, Taylor C, Burchill SA (2007) Chromosome 9p21 gene copy number and prognostic significance of p16 in ESFT. Br J Cancer 96: 1914–1923.
Burchill SA (2003) Ewing’s sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol 56: 96–102.
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, Stoter G, Nooter K (2003) RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res 9: 827–836.
Cai X, Wang T, Shi L (1999) [Expression and clinical implication of multidrug resistance gene and multidrug resistant-associated protein gene in patients with hypopharyngeal squamous cell carcinoma]. Zhonghua Er Bi Yan Hou Ke Za Zhi 34: 173–176.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258: 1650–1654.
Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, Craft AW (2000) Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol 18: 3108–3114.
Dalal S, Berry AM, Cullinane CJ, Mangham DC, Grimer R, Lewis IJ, Johnston C, Laurence V, Burchill SA (2005) Vascular endothelial growth factor: a therapeutic target for tumors of the Ewing's sarcoma family. Clin Cancer Res 11: 2364–2378.
Devine SE, Hussain A, Davide JP, Melera PW (1991) Full length and alternatively spliced pgp1 transcripts in multidrug-resistant Chinese hamster lung cells. J Biol Chem 266: 4545–4555.
Engel T, Bode G, Lueken A, Knop M, Kannenberg F, Nofer JR, Assmann G, Seedorf U (2006) Expression and functional characterization of ABCG1 splice variant ABCG1(666). FEBS Lett 580: 4551–4559.
Fernetti C, Pascolo L, Podda E, Gennaro R, Stebel M, Tiribelli C (2001) Preparation of an antibody recognizing both human and rodent MRP1. Biochem Biophys Res Commun 288: 1064–1068.
Filipits M, Suchomel RW, Zochbauer S, Brunner R, Lechner K, Pirker R (1997) Multidrug resistance-associated protein in acute myeloid leukemia: no impact on treatment outcome. Clin Cancer Res 3: 1419–1425.
Fischer M, Skowron M, Berthold F (2005) Reliable transcript quantification by real-time reverse transcriptase-polymerase chain reaction in primary neuroblastoma using normalization to averaged expression levels of the control genes HPRT1 and SDHA. J Mol Diagn 7: 89–96.
Gaspar N, Rey A, Berard PM, Michon J, Gentet JC, Tabone MD, Roche H, Defachelles AS, Lejars O, Plouvier E, Schmitt C, Bui B, Boutard P, Taque S, Munzer M, Vannier JP, Plantaz D, Enz-Werle N, Oberlin O (2012) Risk adapted chemotherapy for localised Ewing's sarcoma of bone: the French EW93 study. Eur J Cancer 48: 1376–1385.
Grant CE, Kurz EU, Cole SP, Deeley RG (1997) Analysis of the intron-exon organization of the human multidrug-resistance protein gene (MRP) and alternative splicing of its mRNA. Genomics 45: 368–378.
Haber M, Bordow SB, Haber PS, Marshall GM, Stewart BW, Norris MD (1997) The prognostic value of MDR1 gene expression in primary untreated neuroblastoma. Eur J Cancer 33: 2031–2036.
Haber M, Smith J, Bordow SB, Flemming C, Cohn SL, London WB, Marshall GM, Norris MD (2006) Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J Clin Oncol 24: 1546–1553.
He X, Ee PL, Coon JS, Beck WT (2004) Alternative splicing of the multidrug resistance protein 1/ATP binding cassette transporter subfamily gene in ovarian cancer creates functional splice variants and is associated with increased expression of the splicing factors PTB and SRp20. Clin Cancer Res 10: 4652–4660.
Hipfner DR, Almquist KC, Leslie EM, Gerlach JH, Grant CE, Deeley RG, Cole SP (1997) Membrane topology of the multidrug resistance protein (MRP). A study of glycosylation-site mutants reveals an extracytosolic NH2 terminus. J Biol Chem 272: 23623–23630.
Huang M, Lucas K (2011) Current therapeutic approaches in metastatic and recurrent ewing sarcoma. Sarcoma 2011: 863210.
Izquierdo MA, van der Zee AG, Vermorken JB, van der Valk P, Belien JA, Giaccone G, Scheffer GL, Flens MJ, Pinedo HM, Kenemans P, Meijer CJLM, de Vries EGE, Scheper RJ (1995) Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. J Natl Cancer Inst 87: 1230–1237.
Kantharidis P, El-Osta A, deSilva M, Wall DM, Hu XF, Slater A, Nadalin G, Parkin JD, Zalcberg JR (1997) Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin Cancer Res 3: 2025–2032.
Knoop LL, Baker SJ (2001) EWS/FLI alters 5′-splice site selection. J Biol Chem 276: 22317–22322.
Kourti M, Vavatsi N, Gombakis N, Sidi V, Tzimagiorgis G, Papageorgiou T, Koliouskas D, Athanassiadou F (2007) Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int J Hematol 86: 166–173.
Krishnamachary N, Ma L, Zheng L, Safa AR, Center MS (1994) Analysis of MRP gene expression and function in HL60 cells isolated for resistance to adriamycin. Oncol Res 6: 119–127.
Kutanzi KR, Yurchenko OV, Beland FA, Checkhun VF, Pogribny IP (2011) MicroRNA-mediated drug resistance in breast cancer. Clin Epigenetics 2: 171–185.
Lacueva J, Perez-Ramos M, Soto JL, Oliver I, Andrada E, Medrano J, Perez-Vazquez T, Arroyo A, Carrato A, Ferragut JA, Calpena R (2005) Multidrug resistance-associated protein (MRP1) gene is strongly expressed in gastric carcinomas. Analysis by immunohistochemistry and real-time quantitative RT-PCR. Histopathology 46: 389–395.
Lastowska M, Viprey V, Santibanez-Koref M, Wappler I, Peters H, Cullinane C, Roberts P, Hall AG, Tweddle DA, Pearson AD, Lewis I, Burchill SA, Jackson MS (2007) Identification of candidate genes involved in neuroblastoma progression by combining genomic and expression microarrays with survival data. Oncogene 26: 7432–7444.
London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM, Reynolds CP, Cohn SL (2005) Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol 23: 6459–6465.
Lu QJ, Dong F, Zhang JH, Li XH, Ma Y, Jiang WG (2004) Expression of multidrug resistance-related markers in primary neuroblastoma. Chin Med J (Engl) 117: 1358–1363.
Lukas J, Gao DQ, Keshmeshian M, Wen WH, Tsao-Wei D, Rosenberg S, Press MF (2001) Alternative and aberrant messenger RNA splicing of the mdm2 oncogene in invasive breast cancer. Cancer Res 61: 3212–3219.
Maniatis T, Tasic B (2002) Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418: 236–243.
Maraldi NM, Zini N, Santi S, Scotlandi K, Serra M, Baldini N (1999) P-glycoprotein subcellular localization and cell morphotype in MDR1 gene-transfected human osteosarcoma cells. Biol Cell 91: 17–28.
Mignogna C, Staibano S, Altieri V, De Rosa G, Pannone G, Santoro A, Zamparese R, D'Armiento M, Rocchetti R, Mezza E, Nasti M, Strazzullo V, Montanaro V, Mascolo M, Bufo P (2006) Prognostic significance of multidrug-resistance protein (MDR-1) in renal clear cell carcinomas: a five year follow-up analysis. BMC Cancer 6: 293.
Molinari A, Calcabrini A, Meschini S, Stringaro A, Crateri P, Toccacieli L, Marra M, Colone M, Cianfriglia M, Arancia G (2002) Subcellular detection and localization of the drug transporter P-glycoprotein in cultured tumor cells. Curr Protein Pept Sci 3: 653–670.
Nakanishi T, Bailey-Dell KJ, Hassel BA, Shiozawa K, Sullivan DM, Turner J, Ross DD (2006) Novel 5' untranslated region variants of BCRP mRNA are differentially expressed in drug-selected cancer cells and in normal human tissues: implications for drug resistance, tissue-specific expression, and alternative promoter usage. Cancer Res 66: 5007–5011.
Nooter K, Westerman AM, Flens MJ, Zaman GJ, Scheper RJ, van Wingerden KE, Burger H, Oostrum R, Boersma T, Sonneveld P, Gratama JW, Kok T, Eggermont AMM, Bosman FT, Stoter G. (1995) Expression of the multidrug resistance-associated protein (MRP) gene in human cancers. Clin Cancer Res 1: 1301–1310.
Norris MD, Bordow SB, Marshall GM, Haber PS, Cohn SL, Haber M (1996) Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med 334: 231–238.
Nuti SL, Mehdi A, Rao US (2000) Activation of the human P-glycoprotein ATPase by trypsin. Biochemistry 39: 3424–3432.
Oda Y, Dockhorn-Dworniczak B, Jurgens H, Roessner A (1997) Expression of multidrug resistance-associated protein gene in Ewing's sarcoma and malignant peripheral neuroectodermal tumor of bone. J Cancer Res Clin Oncol 123: 237–239.
Panyam J, Labhasetwar V (2003) Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res 20: 212–220.
Perri T, Fogel M, Mor S, Horev G, Meller I, Loven D, Issakov J, Kollender Y, Smirnov A, Zaizov R, Cohen IJ (2001) Effect of P-glycoprotein expression on outcome in the Ewing family of tumors. Pediatr Hematol Oncol 18: 325–334.
Plasschaert SL, de Bont ES, Boezen M, vander Kolk DM, Daenen SM, Faber KN, Kamps WA, de Vries EG, Vellenga E (2005) Expression of multidrug resistance-associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clin Cancer Res 11: 8661–8668.
Qiu YY, Mirkin BL, Dwivedi RS (2007) MDR1 hypermethylation contributes to the progression of neuroblastoma. Mol Cell Biochem 301: 131–135.
Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, Couraud PO, Roux F (1998) Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem 71: 705–715.
Robin TP, Smith A, McKinsey E, Reaves L, Jedlicka P, Ford HL (2012) EWS/FLI1 regulates EYA3 in Ewing sarcoma via modulation of miRNA-708, resulting in increased cell survival and chemoresistance. Mol Cancer Res 10: 1098–1108.
Rodriguez-Galindo C, Navid F, Liu T, Billups CA, Rao BN, Krasin MJ (2007) Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 19: 814–820.
Roessner A, Ueda Y, Bockhorn-Dworniczak B, Blasius S, Peters A, Wuisman P, Ritter J, Paulussen M, Jurgens H, Bocker W (1993) Prognostic implication of immunodetection of P glycoprotein in Ewing’s sarcoma. J Cancer Res Clin Oncol 119: 185–189.
Romani M, Tonini GP, Banelli B, Allemanni G, Mazzocco K, Scaruffi P, Boni L, Ponzoni M, Pagnan G, Raffaghello L, Ferrini S, Croce M, Casciano I (2003) Biological and clinical role of p73 in neuroblastoma. Cancer Lett 197: 111–117.
Roundhill EA, Burchill SA (2012) Detection and characterisation of multi-drug resistance protein 1 (MRP-1) in human mitochondria. Br J Cancer 106: 1224–1233.
Sanchez G, Delattre O, Auboeuf D, Dutertre M (2008) Coupled alteration of transcription and splicing by a single oncogene: boosting the effect on cyclin D1 activity. Cell Cycle 7: 2299–2305.
Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T (2005) MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol 128: 324–332.
Scotlandi K, Remondini D, Castellani G, Manara MC, Nardi F, Cantiani L, Francesconi M, Mercuri M, Caccuri AM, Serra M, Knuutila S, Picci P (2009) Overcoming resistance to conventional drugs in Ewing sarcoma and identification of molecular predictors of outcome. J Clin Oncol 27: 2209–2216.
Seidel A, Hasmann M, Loser R, Bunge A, Schaefer B, Herzig I, Steidtmann K, Dietel M (1995) Intracellular localization, vesicular accumulation and kinetics of daunorubicin in sensitive and multidrug-resistant gastric carcinoma EPG85-257 cells. Virchows Arch 426: 249–256.
Serlo JA, Helenius IJ, Sampo M, Vettenranta K, Saarinen-Pihkala UM, Kivivuori SM, Riikonen P, Kivioja A, Bohling T, Kallajoki M, Ristimaki A, Vasama K, Tarkkanen M (2012) Ewing’s sarcoma family of tumors in Finland during 1990-2009: A population-based study. Acta Oncol 52: 767–775.
Stahl M, Ranft A, Paulussen M, Bolling T, Vieth V, Bielack S, Gortitz I, Braun-Munzinger G, Hardes J, Jurgens H, Dirksen U (2011) Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 57: 549–553.
Styczynski J, Wysocki M, Debski R, Czyzewski K, Kolodziej B, Rafinska B, Kubicka M, Koltan S, Koltan A, Pogorzala M, Kurylak A, Olszewska-Slonina D, Balwierz W, Juraszewska E, Wieczorek M, Olejnik I, Krawczuk-Rybak M, Kuzmicz M, Kowalczyk J, Stefaniak J, Badowska W, Sonta-Jakimczyk D, Szczepanski T, Matysiak M, Malinowska I, Stanczak E, Wachowiak J, Konatkowska B, Gil L, Balcerska A, Maciejka-Kapuscinska L (2007) Predictive value of multidrug resistance proteins and cellular drug resistance in childhood relapsed acute lymphoblastic leukemia. J Cancer Res Clin Oncol 133: 875–893.
Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suva D, Clement V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I (2009) Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res 69: 1776–1781.
Yang L, Chansky HA, Hickstein DD (2000a) EWS.Fli-1 fusion protein interacts with hyperphosphorylated RNA polymerase II and interferes with serine-arginine protein-mediated RNA splicing. J Biol Chem 275: 37612–37618.
Yang L, Embree LJ, Hickstein DD (2000b) TLS-ERG leukemia fusion protein inhibits RNA splicing mediated by serine-arginine proteins. Mol Cell Biol 20: 3345–3354.
Yang X, Jia L, Wei L, Zuo W, Song S (2002) [Correlation of expression levels of multidrug resistance gene 1 (mdr1) mRNA, multidrug resistance-associated protein (MRP), amd P-glycoprotein (P-gp) with chemotherapy efficacy in malignant lymphomas]. Zhonghua Yi Xue Za Zhi 82: 1177–1179.
Yonemori K, Yamaguchi U, Kaneko M, Uno H, Takeuchi M, Ando M, Fujiwara Y, Hosono A, Makimoto A, Hasegawa T, Yokoyama R, Nakatani F, Kawai A, Beppu Y, Chuman H (2007) Prediction of response and prognostic factors for Ewing family of tumors in a low incidence population. J Cancer Res Clin Oncol 134: 389–395.
Zhou SF (2008) Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 38: 802–832.
Acknowledgements
We thank the Children’s Cancer and Leukaemia Group (CCLG) Tumour Bank for providing tumour samples and clinical outcome information. We also thank Dr Catherine Cullinane (Paediatric Pathologist, St James’s University Hospital) for examination of tumour samples by microscopy. This work was supported by a grant from the Yorkshire Cancer Research (YCR, grant number L352) to SAB. We thank Mr Colin Johnston and Professor Tim Bishop (Cancer Research UK Clinical Centre, Leeds, UK) for performing statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
There authors declare no conflicts of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Roundhill, E., Burchill, S. Membrane expression of MRP-1, but not MRP-1 splicing or Pgp expression, predicts survival in patients with ESFT. Br J Cancer 109, 195–206 (2013). https://doi.org/10.1038/bjc.2013.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.168
Keywords
This article is cited by
-
ABCA6 affects the malignancy of Ewing sarcoma cells via cholesterol-guided inhibition of the IGF1R/AKT/MDM2 axis
Cellular Oncology (2022)
-
New Medical/Biologic Paradigms in the Treatment of Bone Tumors
Current Surgery Reports (2014)