Abstract
Study design: Case report.
Objective: To report a rare cause of spinal cord compression.
Setting: University Hospital, Wales, UK.
Case report: A 67-year-old gentleman on oral anticoagulation for atrial fibrillation presented with a 4-h history of progressive loss of sensation and weakness in both legs; there was no history of trauma. On examination, he had a flaccid paraplegia with altered sensation in the L1,2,3 dermatomes and complete anaesthesia in the L4,5 distribution. Knee and ankle jerk reflexes were absent, plantars were equivocal and anal sphincter tone was reduced. The patient's international normalized ratio (INR) was 4.1. An MR scan showed an extensive intradural haematoma compressing the cauda equina. The anticoagulation was reversed and an urgent T12-L2 laminectomy was performed; findings were a circumferential haematoma at L1 extending in the anterior canal between T10 and L3. The patient had an uneventful postoperative course generally, but at 1 week there was no neurological recovery.
Conclusion: This case highlights that anticoagulation even when well controlled is not without risk. This is particularly of concern as the number of patients receiving long-term anticoagulation therapy has doubled between 1993 and 1998.
Similar content being viewed by others
Introduction
Spinal subdural haematoma is a rare cause of spinal cord compression. First described by Potts in 1910, it is generally associated with excess anticoagulation or coagulopathy with minor trauma. Spontaneous subdural haematoma (in the absence of any of these factors) has been recorded six times.1 This report describes a case of extensive acute subdural haematoma in a patient who was anticoagulated with warfarin with an international normalized ratio (INR) of 4.1.
Case report
A 67-year-old gentleman presented to the Accident and Emergency Department. He had woken at 0700 that day and noted a lack of normal sensation when passing urine and the gradual onset of increasing lumbar pain. He lay flat on a sofa and experienced a progressive loss of sensation in his legs so that on presentation at 1100, he was unable to move his legs. He had not passed urine since 0700. There was no history of trauma, but there was a long history of episodic mechanical backache with no recent exacerbations. He had atrial fibrillation treated by warfarin for 15 years with a record of good control in the range (INR) 2.0–3.0. In 1947, a splenectomy was performed following abdominal trauma. He had benign prostatic hypertrophy for which a transurethral resection had been performed and he reported no recent symptoms.
On neurological examination, there was flaccid paraplegia with neither muscle wasting nor fasciculation. Knee and ankle reflexes were absent and plantars were equivocal. Sensory testing revealed an altered quality of light-touch in the dermatomes of L1, 2 and 3, and complete anaesthesia in L4–S5 distribution, which includes the perianal and perineal areas. Anal sphincter tone was reduced.
General examination was otherwise unremarkable.
A full blood count (FBC) showed a microcytic hypochromic anaemia, haemoglobin 11.8 g/dl (13.0–16.5), MCV 76.1 fl (84.0–99.0), WBC 13.44 × 109/l (4.0–10.5), platelets 429 × 109/l (150–400). The INR was 4.1. Quality assurance of prothrombin time estimation, from which INR is calculated, in our laboratory was verified throughout the day by running normal and abnormal controls. The laboratory also participates in external quality assurance in line with accepted UK practice.
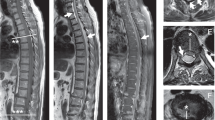
An MR scan of the thoracolumbar spine was abnormal and demonstrated an extensive intradural haematoma causing compression of the cauda equina (Figures 1 and 2).
The patient was immediately prepared for theatre with urgent reversal of the anticoagulation using vitamin K and fresh frozen plasma to INR 1.6. At 2200 under general anaesthetic, a T10 – L2 laminectomy was performed. The haematoma was found to be circumferential at L1, but was otherwise limited to the anterior canal extending three levels cephalad to (T10) and a single level caudally to (L3).
The patient had an uneventful postoperative course generally, but at 1 week there was no neurological recovery, with a flaccid paraplegia; an L1 sensory level, incontinence of faeces and loss of conscious detrusor control remained.
Discussion
The use of anticoagulation in the treatment and prophylaxis of a variety of medical disorders has been long established. The indications for anticoagulation vary from the prophylactic use in atrial fibrillation and prosthetic heart valve to the treatment of deep-vein thrombosis and pulmonary embolism. With the known risk of bleeding while on treatment, INR monitoring is mandatory. In a meta-analysis of five randomized control trials the risk of haemorrhage, of any kind, associated with anticoagulation has been reported to be as low as 1.3% per treatment, even at the upper end of the therapeutic range.2 However, at any point in time, only half the anticoagulated patients are within therapeutic range.3 A recent report has documented an excess mortality, from bleeding, associated with high INR values and advocates less intensive treatment with INRs of 2.2–2.3 irrespective of the indication for treatment.4
The latest (1998) guidelines from the British Committee for Standards in Haematology (BCSH) set a target INR for each indication allowing a variation of 0.5 units above or below this figure.5 Previous studies of patients anticoagulated for atrial fibrillation have stated target INRs ranging from 1.5 to 4.5.5 The new guidelines set the target for atrial fibrillation as 2.5. The observed INR of 4.1 in this case was well above this current target. However, a target INR of 3.5 is recommended for three conditions: mechanical prosthetic heart valve, recurrent venous thromboembolism on warfarin and antiphospholipid syndrome. For any of these indications, an INR of 4.1 is just 0.1 units above the therapeutic range, which is within the accepted range of accuracy for INR measurement.
Spinal subdural haematoma has been reported in the literature in two groups, the first more common type associated with excess anticoagulation or coagulaopathy and minor trauma. In this context, trauma may include lumbar puncture, epidural anaesthesia and other medical procedures. In the second, very rare, group there is no identified predisposing factor and the onset is said to be ‘spontaneous’. The patient reported in this case certainly had no history of trauma or coagulopathy, but strictly speaking, was overanticoagulated as his target INR was 2.5. However, this INR would not be unreasonable for the three conditions listed above and hence, this presentation could be considered spontaneous.
Spontaneous subdural haematomas have been reported in all three spinal sections in a variety of age groups.1 The spinal dura differs anatomically from its cranial extension in its relative distribution of significantly sized bridging veins. Numerous major bridging veins exist in the spinal subarachnoid space, whereas they are absent from the spinal intradural space. One of the theories for the formation of spinal intradural haematomas suggests that they originate in the subarachnoid space, and then extend subdurally.6 It would suggest that the spinal subarachnoid vessels are subject to substantial and rapid fluctuations in pressure transmitted from the thorax and abdomen, these fluctuations being further pronounced by exertion or trauma. During these fluctuations, there is a momentary lag in CSF pressure allowing a pressure gradient, which causes vascular rupture.7 As the subarachnoid blood redistributes within the CSF, the subdural haemorrhage causes cord compression. This is further supported by the finding of a case of spontaneous chronic SDH associated with a cervical neurilemmoma in a child.8 Spinal neurilemmomas are contained entirely within the subarachnoid space. If it can be assumed that the haemorrhage originated from the tumour (ie the two events were not synchronous), then blood must have traversed the arachnoid from the subarachnoid to the subdural space.
As subdural haematomas are uncommon, data on outcome are sparse. Groen and van Alpen9 have reviewed 330 reported cases of spontaneous spinal epidural haematomas, showing that degrees of neurological deficit and operative interval are critical factors in determining outcome. Postoperative neurological condition was significantly better for complete sensorimotor deficit when decompression was performed within 36 h. Lack of recovery in this case points to a more sinister prognosis than for an epidural haematoma and the urgent need for surgical treatment. There was a delay in the surgical management of this case and this may have adversely affected the outcome. Cauda equina compression by the haematoma was present in excess of 12 h prior to drainage and substantial recovery could not be expected at this time.
In the UK, the number of patients receiving long-term anticoagulation therapy doubled between 1993 and 1998.5 This case illustrates that anticoagulation, even when controlled, is not without risk and that this may have catastrophic consequences.
References
Calhoun JM, Boop F . Spontaneous spinal subdural haematoma: case report and review of the literature. Neurosurgery 1991; 29: 133–134.
Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154: 1449–1457.
Rose P . Audit of anticoagulant therapy. J Clin Pathol 1996; 49: 5–9.
Odén A, Fahlén M . Oral anticoagulation and risk of death: a medical report linkage study. Br Med J 2002; 325: 1073–1075.
British Committee for Standards in Haematology. BCSH Guidelines on oral anticoagulation, 3rd ed. Br J Haematol 1998; 101: 37–387.
Swann KW, Ropper AH, New PFJ, Poletti CE . Spontaneous spinal subarachnoid haemorrhage and subdural haematoma. J Neurosurg 1984; 61: 975–980.
Rader JP . Chronic subdural haematoma of the spinal canal: report of a case. N Eng J Med 1955; 253: 374–376.
Smith RA . Spinal subdural haematoma, neurilemmoma and acute transverse myelopathy. Surg Neurol 1985; 23: 367–370.
Groen RJM, van Alpen HAM . Operative treatment of spontaneous spinal epidural haematomas: a study of the factors determining postoperative outcome. Neurosurgery 1996; 39: 494–508.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miller, D., Ray, A. & Hourihan, M. Spinal subdural haematoma: how relevant is the INR?. Spinal Cord 42, 477–480 (2004). https://doi.org/10.1038/sj.sc.3101591
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101591