Abstract
Introduction
Holospinal epidural abscess (HEA) extending from the cervical to the lumbosacral spine is an extremely rare condition. Surgical treatment of HEA, which involves extensive decompression of the spinal lesion is difficult in emergency settings. However, the authors successfully treated a case of HEA in critical condition with severe neurological deficits through a combination of skip decompression surgeries and catheter irrigation.
Case presentation
A 73-year-old man complained of neck and back pain and developed muscle weakness in the upper and lower extremities (C5 AIS D tetraplegia). When he was transferred to our hospital, a marked increase in leukocytes (13330/μL) and C-reactive protein levels (32.11 mg/dL) was observed. Magnetic resonance imaging (MRI) revealed a HEA extending from C1 to S2 levels. Therefore, an emergency posterior decompression on C4-5 and T4-7 was performed, followed by catheter irrigation using a venous catheter. Blood and intraoperative isolated microorganisms were identified as Streptococcus intermedius, which is a rare cause of spinal infection. He experienced marked improvement in pain after surgery. Two months after surgery, the epidural abscess completely disappeared. Motor weakness gradually improved, and he was able to walk without support and showed no pain recurrence during the final follow-up (20 months after surgery).
Discussion
Early diagnosis is important for the treatment of HEAs. Therefore, a whole spine MRI is recommended when an extensive spinal epidural abscess is suspected. Decompression surgery at limited spine levels followed by catheter irrigation should be considered in patients with HEA.
Similar content being viewed by others
Introduction
Pyogenic spondylitis (PS) is a non-specific and acute or subacute infection involving the intervertebral disks and adjacent vertebral bodies. PS predominantly occurs in the elderly and chronically debilitated patients over 50 years of age, further increasing in elderly populations [1]. Patients with PS often have systemic disorders such as diabetes mellitus, chronic renal failure, cancer, and immunocompromised status [2]. In some patients, spinal epidural abscess (SEA) accompanies PS. However, SEA is not a common condition with an estimated incidence of 0.2–2.0/10,000 hospital admissions [2, 3]. Although some cases without neurological deficits can be treated conservatively with empirical antimicrobial therapy, this treatment sometimes fails or usually requires a long period of time [4]. In patients unresponsive to conservative treatment, patients’ symptoms usually worsen due to biomechanical instability caused by bony destruction and/or neurological impairment resulting from SEA in about 1/3 of all cases [5, 6]. In cases of neurological deficits, surgical treatment is indicated at the appropriate time [7], and emergency evacuation of the abscess with decompression of the spinal cord and nerve roots can be performed [2].
Holospinal epidural abscess (HEA) extending from the cervical spine to the lumbosacral spine is an extremely rare condition that can lead to major permanent neurological deficits and significant mortality [8]. Surgical treatment of HEA involves extensive decompression from the cervical to the lumbar spinal lesion and is clinically difficult in emergency settings. We report a case of a patient with HEA with a poor general condition and severe neurological deficits who was successfully treated surgically with skip decompression and catheter irrigation.
Case presentation
History
A 73-year-old man with hypertension and diabetes complained of neck and low back pain for 7 days. He had a history of tooth extraction treatment one year before the first presentation. He progressively developed muscle weakness on his upper and lower extremities, and eventually could not walk. The patient was transferred to our hospital because a HEA extending widely from the cervical to sacral regions was detected on magnetic resonance imaging (MRI).
Examination
Upon arrival in the emergency room, the patient’s vital signs were as follows: body temperature, 38.6 °C; blood pressure, 159/95 mmHg; pulse rate, 24 bpm; and Glasgow Coma Scale, E4V5M6. However, he was drowsy and unable to communicate promptly.
Upon examination, severe cervical and low back pain were observed, and deep tendon reflexes on either the upper or lower extremities were present. Although the accurate neurological evaluation was difficult because of communication difficulties, weakness of both the upper and lower extremities (Medical Research Council grade 2–3/5, C5 AIS D tetraplegia), and sensory decline below T4 dermatome was observed.
Pathological findings
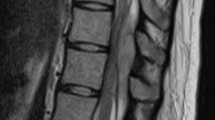
Laboratory test results showed a marked increase in leukocytes (13330/μL), C-reactive protein (CRP; 32.11 mg/dL)levels, and procalcitonin (PCT; 4.40 ng/mL) levels, suggesting that a severe infection was present. Blood glucose was 126 mg/dL, and HbA1c was 6.4%, suggesting that diabetes mellitus was controlled. Plain radiographs of the whole spine showed no obvious pathologic findings except for an erosion of the vertebral endplates at the L3-4 level. MRI of the cervical to lumbar regions was taken, and fluid accumulation in the dorsal portion of the epidural space from the C1 to S2 levels was detected. The fluid accumulation showed iso-intensity with peripheral enhancement by gadolinium-enhanced T1-weighted images, high-intensity T2-weighted, and short tau inversion recovery (STIR) images, suggesting a diagnosis of HEA at that time (Fig. 1). The fluid compressed the spinal cord and was considered as the main cause of the patient’s neurological symptoms, particularly, at the most severely compressed levels of the spinal cord, T3-8 (Fig. 1). The intervertebral disks and surrounding vertebral endplates showed high intensity on T2-weighted images of L3-4, L4-5, and L5-S1 levels, suggesting the presence of PS at these levels.
A T1-weighted sagittal image. White bars indicate C3-4 (*), T6-7 (**), and L3-4 (***) levels, at which axial images are shown in (D), (E), and (F), respectively. B T2-weighted sagittal image. Arrows indicate epidural abscesses. C T1-weighted gadolinium-enhanced image. The arrow indicates epidural abscess which shows peripheral enhancement. D–F T2-weighted axial images at C3-4 (D), T6-7 (E), and L3-4 (F). Arrows indicate a spinal epidural abscess. Asterisks indicate the spinal cord (D, E) or cauda equina (F) compressed by a huge epidural abscess located in the dorsal portion of the spinal canal.
Operation
The patient’s general condition was critical and he was a high-risk surgical candidate. Paralysis of the upper and lower extremities was progressive, and C1-S2 HEA was the definitive cause of that paralysis. Thus, urgent surgery with abscess drainage was performed. Considering the invasiveness of the procedure, decompression of all segments from C1 to S2 could not be performed. The most compressed spinal levels were selected to be surgically treated. We performed C4-5 open-door laminoplasty and T4-7 laminectomy, and then yellowish-white fluid was observed in the epidural space (Fig. 2). Samples were collected and sent for Gram staining and bacteriological culture. Abscess adhering to the dura was washed with saline using a 16-gauge venous catheter inserted into the cranial and caudal sides of the decompressed sites. Irrigation and evacuation procedures were continued until no abscess or purulent materials were observed (Fig. 2).
A Epidural abscess is exposed after T4-7 laminectomy. Membranous tissue on the surface of the epidural abscess is separated (arrow in (A)), then, (B) yellowish-white abscess comes out (arrow in (B)). C After the resection of the abscess, a venous catheter (arrow) is inserted into the cranial edge of the decompressed spinal canal (*). Saline is infused into the non-decompressed spinal canal to wash out the abscess. D Epidural abscess is washed away, and the spinal canal (T4-7) is completely decompressed (arrows).
Postoperative course
Intravenous antibiotic treatment with meropenem (3.0 g/day) and vancomycin (2.0 g/day) was started before surgery. The results of Gram stain examination of surgical tissue sample (epidural abscess) was gram-positive cocci; therefore, intravenous antibiotic treatment with meropenem (6.0 g/day) and vancomycin (2.0 g/day) was continued. on the first postoperative day, leukocyte count and CRP dropped to 11000/μL and 13.75 mg/dL, respectively. Three days after surgery, the daily dose of vancomycin was increased to 2.5 g/day, based on serum vancomycin trough level. Nine days after surgery, it was confirmed that blood cultures were positive for Streptococcus intermedius, and the antibiotic was switched to ampicillin (8.0 g/day) according to in vitro sensitivity results. Streptococcus intermedius is a species of resident bacteria in the oral cavity. PCT was normalized (<0.5 ng/mL) 9 days after surgery, and leukocyte count was normalized (<9000/μL) 14 days after surgery. Twenty days after surgery, improvement in CRP was not sufficient (5.62 mg/dL). Therefore, 21 days after surgery, the antibiotic was switched again to ceftriaxone sodium (4.0 g/day). Forty-three days after surgery, CRP levels had dropped to 1.08 mg/dL, and intravenous antibiotic treatment was stopped, followed by oral antibiotic treatment using sulfamethoxazole (1.6 g/day), trimethoprim (320 mg/day), and minocycline (200 mg/day) until 52 days after surgery. Since the effect was inadequate, the antibiotic was switched to levofloxacin (500 mg/day) until 62 days after surgery, followed by sitafloxacin hydrate(100 mg/day). Eighty-five days after surgery, the patient was discharged from our hospital and moved to another hospital to receive nursing care. Due to the onset of severe diarrhea, oral administration was discontinued after 92 days. Eventually, 187 days after the surgery, CRP levels dropped to the normal range (0.29 mg/dL). Detailed time courses of antibiotic treatment, CRP levels, leukocyte counts, and PCT were described in Fig. 3.
The horizontal axis indicates the days before (minus) and after surgery, and the vertical axis indicates the levels of CRP, WBC, and PCT. The gray horizontal bars below the graph indicate the intravenous antibiotic treatment. The white horizontal bars extending from the gray bars indicate oral antibiotic treatment. MEPM meropenem, VCM vancomycin, ABPC ampicillin, CTRX ceftriaxone sodium, SMX sulfamethoxazole, TMP trimethoprim, MINO minocycline, LVFX levofloxacin, STFX sitafloxacin hydrate.
One week after surgery, MRI showed decreased epidural dorsal abscess volumes at the cervical and thoracic spine levels. After 2 months, the abscess completely disappeared at all levels of the spine (Fig. 4). MRI was performed at the final follow-up 20 months after surgery, showing no recurrence of the epidural abscess (Fig. 4).
A T2-weighted sagittal image of the whole spine taken 2 months after surgery. No spinal epidural abscess is observed. White bars indicate C3-4 (*), T6-7 (**), and L3-4 (***) levels, at which axial images are shown in (B), (C), and (D), respectively. B–D Epidural abscess completely disappears on T2-weighted axial images. E T2-weighted sagittal image of the whole spine taken 20 months after surgery, showing no recurrence of spinal epidural abscess.
He experienced marked improvement in cervical and low back pain immediately after surgery. Motor weakness of the upper and lower extremities gradually improved. He was discharged 17 weeks after surgery and moved to a rehabilitation facility. He was able to walk without support, and no recurrence of infection was observed at the final follow-up 20 months after surgery. Slight numbness in the right finger remained. However, muscle strength in his lower extremities and urinary function had returned to normal.
Discussion
The most common symptoms of SEA are pain, fever, and neurological deficits. However, these symptoms are nonspecific and could lead to a high rate of misdiagnosis [9]. A previous study reported that the rate of misdiagnosis of SEA is ≥50% [10]. Elevated leukocyte count and CRP levels are useful markers for diagnosing epidural abscesses [11]. However, it should be noted that these significant findings may not always be obtained in the early stage of onset [7]. MRI is the best diagnostic tool for visualizing the extent and location of an epidural abscess [2]. Therefore, a whole spine MRI should be performed when SEA is suspected based on patients’ clinical findings [10]. Regarding staging of SEA, Heusner et al. [12]. reported the following: stage I: local back pain; stage II: radiculopathy; stage III: muscle weakness/sensory dysfunction/vesicorectal disorder; and stage IV: complete paralysis. It has been reported that stage III to stage IV periods progress quickly and showed a poorer prognosis. Since early diagnosis is important for the treatment of SEA and HEA based on our experience, we would like to recommend doing an MRI scan in cases of the back (or neck) pain, fever, and neurological deficits as early as possible. In addition, a whole spine MRI scan is recommended when extensive SEA is detected on MRI with a limited area of the spine.
Infectious spinal diseases such as PS, SEA, and HEA are generally recognized as hematogenous infections [13]. It has been widely recognized that Staphylococcus aureus is the most commonly isolated microorganism. However, methicillin-resistant Staphylococcus aureus (MRSA) is isolated from 17.2% of patients with PS [14]. In our case, blood and intraoperative isolated microorganisms were Streptococcus intermedius, which is a rare cause of spinal infection [15]. Streptococcus intermedius is found at various mucosal sites, including the oral cavity, genitourinary system, and gastrointestinal tract [16]. It is also frequently encountered in various infectious diseases, including liver and brain abscesses; dentoalveolar infections; and infective endocarditis [16]. There are few reports of SEA or HEA caused by Streptococcus intermedius. However, our case indicates that it could be a cause of SEA and HEA. Our patient underwent a thorough examination to detect the source of infection; however, no source of Streptococcus intermedius infection was found. The patient underwent tooth extraction treatment one year before the onset of HEA. Although no oral infection was present after admission, we hypothesize that the source of Streptococcus intermedius was the previous infection of the oral cavity.
There are few reports of HEA treated surgically with total laminectomy [17]. When the laminectomy range is widened, operative time would take too long in patients with unstable conditions, and the maximum invasiveness to the posterior elements of the spine may cause spinal instability [18]. To reduce surgical invasion and avoid spinal instability, several reports proposed the advantages of posterior skip decompression surgeries [18]. In addition, the use of a catheter enables wider drainage than that in the range of posterior decompression [19]. Similar to this case, there is a case report describing that skip decompression surgeries with catheter irrigation successfully prevented neurological deterioration and spinal instability [19]. To decide the levels of posterior decompression, a whole spine MRI should be performed. Before surgery, it is necessary to evaluate which spinal level directly corresponds to the patients’ neurological deficits and assess which levels are the best to perform skip decompression surgeries for achieving the most effective drainage.
We encountered a case of HEA with poor general condition and paralysis. Skip decompression surgeries and abscess drainage using a catheter were performed early after the onset of paralysis, resulting in good surgical outcomes. A whole spinal MRI is recommended for early diagnosis and preoperative evaluation. To select the site of skip decompression surgeries by which good surgical results are expected, surgeons should consider multiple preoperative factors such as the patient’s general condition, neurological symptoms, and MRI findings. Because early diagnosis is important to achieve good surgical results for the treatment of HEAs, physicians should be aware of the possibility that epidural abscess can extend from the cervical to the lumbosacral spine. Therefore, a whole spine MRI is recommended when extensive SEA is detected on MRI, particularly in patients with poor general conditions and severe neurological deficits.
References
Fantoni M, Trecarichi EM, Rossi B, Mazzotta V, Di Giacomo G, Nasto LA, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharm Sci. 2012;16:2–7.
Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–12. https://doi.org/10.1136/jnnp.65.2.209.
Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27:177–84.
Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19:575–82. https://doi.org/10.1007/s00586-009-1216-1.
Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3:5–16. https://doi.org/10.1016/j.jiph.2010.01.001.
Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22:2787–99. https://doi.org/10.1007/s00586-013-2850-1.
Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175–204. https://doi.org/10.1007/pl00011954.
Tahir MZ, Hassan RU, Enam SA. Management of an extensive spinal epidu-ral abscess from C-1 to the sacrum. Case report J Neurosurg Spine. 2010;13:780–3. https://doi.org/10.3171/2010.5.SPINE09545.
Alerhand S, Wood S, Long B, Koyfman A. The time-sensitive challenge of diagnosing spinal epidural abscess in the emergency department. Intern Emerg Med. 2017;12:1179–83. https://doi.org/10.1007/s11739-017-1718-5.
Yang X, Guo R, Lv X, Lai Q, Xie B, Jiang X, et al. Challenges in diagnosis of spinal epidural abscess: a case report. Medicine. 2019;98:e14196. https://doi.org/10.1097/MD.0000000000014196.
Torrie PAG, Leonidou A, Harding IJ, Wynne Jones G, Hutchinson MJ, Nelson IW. Admission inflammatory markers and isolation of a causative organism in patients with spontaneous spinal infection. Ann R Coll Surg Engl. 2013;95:604–8. https://doi.org/10.1308/003588413x13629960049351.
Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845–54. https://doi.org/10.1056/NEJM194812022392301.
Bluman EM, Palumbo MA, Lucas PR. Spinal epidural abscess in adults. J Am Acad Orthop Surg 2004;12:155–63. https://doi.org/10.5435/00124635-200405000-00003.
Pola E, Taccari F, Autore G, Giovannenze F, Pambianco V, Cauda R, et al. Multidisciplinary management of pyogenic spondylodiscitis: epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J 2018;27:229–36. https://doi.org/10.1007/s00586-018-5598-9.
Ramhmdani S, Bydon A. Streptococcus intermedius: an unusual cause of spinal epidural abscess. J Spine Surg. 2017;3:243–9. https://doi.org/10.21037/jss.2017.05.04.
Vajramani GV, Nagmoti MB, Patil CS. Neurobrucellosis presenting as an intra-medullary spinal cord abscess. Ann Clin Microbiol Antimicrob. 2005;4:14 https://doi.org/10.1186/1476-0711-4-14.
Gorchynski J, Huang J, McLaughlin T. A methicillin-resistant Staphylococcus aureus-positive holospinal epidural abscess. Am J Emerg Med. 2009;27:514.e7–9. https://doi.org/10.1016/j.ajem.2008.07.041.
Kelly JB, Khoi DT. Holospinal epidural abscesses—institutional experience. J Clin Neurosci. 2018;48:18–27. https://doi.org/10.1016/j.jocn.2017.10.057.
Siasios ID, Fitiadou A, Fountas K, Dimopoulous V. Holospinal epidural abscess in elderly patient. A case presentation and review. Surg Neurol Int. 2019;10:204. https://doi.org/10.25259/SNI_436_2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koyama, K., Aoki, Y., Inoue, M. et al. Skip decompression surgeries in the treatment of holospinal epidural abscess: a case report. Spinal Cord Ser Cases 7, 38 (2021). https://doi.org/10.1038/s41394-021-00401-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00401-w