Abstract
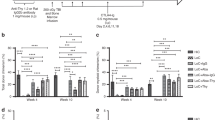
Long-term multilineage allochimerism can be obtained in H2-mismatched B6.SJL to BALB/c transplants with host irradiation of 100 cGy, donor spleen cell pre-exposure and costimulator blockade with anti-CD40 ligand (CD40L) antibody. We evaluated this allochimerism approach in murine marrow transplants with different degrees of major histocompatibility complexe (MHC) mismatching; these include: (1) H2-mismatched transplant H2Kk to H2Kb, (2) full haplo-identical transplant H2Kbd to H2Kbk, (3) a partial haplo-identical transplant H2Kd to H2Kbd and (4) an MHC class II mismatch. Levels of chimerism increased up to 12 weeks and then stayed relatively stable up to 1 year after transplant. At 18 weeks post-transplant, the H2-mismatched, haplo-identical, partial haplo-identical and class II-mismatch transplants evidenced 17.9±4.4, 40.7±0.9, 25.1±4.19 and 33.7±3.5% donor chimerism, respectively. Dropping the anti-CD40 antibody treatment and spleen cells or changing the schedule of antibody to one injection, in haplo-identical or full-mismatched transplants resulted in no donor-derived chimerism. On the other hand, these still resulted in minor chimerism in class II-mismatched transplants. Lineage analysis of peripheral blood at 6 and 12 months post-transplant demonstrated a significant shift toward increased chimeric lymphocytes and decreased chimeric granulocytes in the full H2 as compared with haplo-identical or class II transplants. Transplantation with anti-CD40L antibody eliminated both graft-versus-leukemia and graft-versus-host disease (GVHD) and delayed lymphocyte infusion did not rescue animals from fatal leukemia. In conclusion, under the conditions of our tolerization regimen, a haplo transplant gives higher engraftment levels than a full H2 mismatch, and despite lower engraftment levels, a class II-mismatched transplant can be successfully accomplished with only 100 cGy and no CD40L blockade.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Stewart FM, Zhong S, Wuu J, Hsieh CC, Nilsson SK, Quesenberry PJ . Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood 1998; 91: 3681–3687.
Sharabi Y, Sachs DM . Mixed chimerism and permanent specific transplantation tolerance induced by a non lethal preparative regimen. J Exp Med 1989; 169: 493–502.
Sykes M, Szot GL, Swenson K, Pearson DA . Induction of high levels of allogeneic hematopoietic reconstitution and donor specific tolerance without myelosuppressive conditioning. Nat Med 1997; 3: 783–787.
Quesenberry PJ, Stewart FM, Becker P, D'Hondt L, Frimberger A, Lambert JF et al. Stem cell engraftment strategies. Ann NY Acad Sci 2001; 938: 54–61; discussion 61–62 (Review).
Stewart FM, Zhong S, Lambert JF, Colvin GA, Abedi M, Dooner MS et al. Host marrow stem cell potential and engraftability at varying times after low-dose whole-body irradiation. Blood 2001; 98: 1246–1251.
Rao SS, Peters SO, Crittenden RB, Stewart FM, Ramshaw HS, Quesenberry PJ. Stem cell transplantation in the normal nonmyeloablated host: relationship between cell dose, schedule, and engraftment. Exp Hematol 1997; 25: 114–121.
Van Bekkum DW . Bone marrow transplantation and partial body shielding for estimating cell survival and repopulation. In: Bond VP, Sugahara T (eds). Comparative Cellular and Species Radiosensitivity. Tokyo, Japan: Igaku Shoin Ltd., 1969.
Glasgow GP, Beetham KL, Mill WB . Dose rate effects on the survival of normal hematopoietic stem cells of BALB/c mice. Int J Radiat Oncol Biol Phys 1983; 9: 557–563.
Cayabyab M, Phillips JH, Lanier LL . CD40 preferentially co-stimulates activation of CD4+ T lymphocytes. J Immunol 1994; 152: 1523–1531.
Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA et al. Expression of functional CD40 by vascular endothelial cells. J Exp Med 1995; 182: 33–40.
Grewal IS, Xu J, Flavell RA . Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature 1995; 378: 617–620.
Taylor PA, Noelle RJ, Blazar BR . CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med 2001; 193: 1311–1318.
Durie FH, Aruffo J, Ledbetter J, Crassi KM, Green WR, Fast LD et al. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chromic forms of graft-versus-host disease. J Clin Invest 1994; 94: 1333–1338.
Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Buhlman J, Xu J, Flavell RA et al. Blockade of CD40 ligand CD40 interaction impairs CD4+ T-cell-mediated alloreactivity by inhibiting mature donor T-cell expansion and function after bone marrow transplantation. J Immunol 1997; 158: 29–39.
Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG et al. Extrathymic T-cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T-cell tolerance. J Exp Med 1998; 187: 2037–2044.
Durham MM, Bingaman AW, Adams AB, Ha J, Waitze SY, Pearson TC et al. Administration of anti-CD40 ligand and donor bone marrow leads to hemopoietic chimerism and donor-specific tolerance without cytoreductive conditioning. J Immunol 2000; 165: 1–4.
Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA et al. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood 2000; 95: 2175–2182.
Fishbein TM, Wang L, Benjamin C, Liu J, Tarcsafalvi A, Leytin A et al. Successful tolerance induction under CD40 ligation in a rodent small bowel transplant model: first report of a study with the novel antibody AH.F5. Transplantation 2002; 73: 1943–1948.
Qian Y, Dana MR . Effect of locally administered anti-CD154 (CD40 ligand) monoclonal antibody on survival of allogeneic corneal transplants. Cornea 2002; 21: 592–597.
Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA 1995; 92: 9560–9564.
Guillot C, Guillonneau C, Mathieu P, Gerdes CA, Menoret S, Braudeau C et al. Prolonged blockade of CD40-CD40 ligand interactions by gene transfer of CD40Ig results in long-term heart allograft survival and donor-specific hyporesponsiveness, but does not prevent chronic rejection. J Immunol 2002; 168: 1600–1609.
Quesenberry PJ, Zhong S, Wang H, Stewart FM . Allogeneic chimerism with low dose irradiation, antigen presensitization and costimulator blockade in H-2 mismatched mice. Blood 2001; 97: 557–564.
Dagmar D, Michael B, Marie R, Wanyung Z, Martha H, William H . et al. CD40 ligand induces an antileukemia immune response in vivo. Blood 2001; 90: 1927–1933.
Tsukada N, Kobata T, Aizawa Y, Yagita H, Okumura K . Graft-versus-leukemia effect and graft-versus-host disease can be differentiated by cytotoxic mechanisms in a murine model of allogeneic bone marrow transplantation. Blood 1999; 93: 2738–2747.
Cuzick JA . Wilcoxon-type test for trend. Statist Med 1985; 4: 87–90.
Tsukada N, Kobata T, Aizawa Y, Yagita H, Okumura K . Graft-versus-leukemia effect and graft-versus-host disease can be differentiated by cytotoxic mechanisms in a murine model of allogeneic bone marrow transplantation. Blood 1999; 93: 2738–2747.
Lambert JF, Colvin GA, Zhong S, Wang H, D'Hondt L, Abedi M et al. H2 mismatched transplantation with repetitive cell infusions and CD40 ligand antibody infusions without myeloablation. Br J Haematol 2002; 119: 155–163.
Ito H, Kurtz J, Shaffer J, Sykes M . CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol 2001; 166: 2970–2981.
Kean LS, Durham MM, Adams AB, Hsu LL, Perry JR, Dillehay D et al. A cure for murine sickle cell disease through stable mixed chimerism and tolerance induction after nonmyeloablative conditioning and major histocompatibility complex-mismatched bone marrow transplantation. Blood 2002; 99: 1840–1849.
Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol 2001; 167: 1103–1111.
Author information
Authors and Affiliations
Additional information
This work was supported by Grants P01-HL-56920, PO1-DK-5022, RO1-DK-49650, R01-DK2742.
Rights and permissions
About this article
Cite this article
Abedi, M., Greer, D., Lambert, J. et al. Tolerance induction by costimulator blockade in 100 cGy treated hosts with varying degrees of genetic disparity. Leukemia 17, 1871–1879 (2003). https://doi.org/10.1038/sj.leu.2403070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403070
Keywords
This article is cited by
-
Transplantation of hematopoietic stem cells for induction of unresponsiveness to organ allografts
Springer Seminars in Immunopathology (2004)