Featured
-
-
Article |
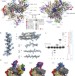 Molecular structures of unbound and transcribing RNA polymerase III
Molecular structures of unbound and transcribing RNA polymerase III
RNA polymerase III (Pol III), the largest eukaryote polymerase yet characterized, transcribes structured small non-coding RNAs; here cryo-electron microscopy structures of budding yeast Pol III allow building of an atomic-level model of the complete 17-subunit complex, both unbound and while elongating RNA.
- Niklas A. Hoffmann
- , Arjen J. Jakobi
- & Christoph W. Müller
-
Letter |
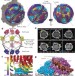 In situ structures of the segmented genome and RNA polymerase complex inside a dsRNA virus
In situ structures of the segmented genome and RNA polymerase complex inside a dsRNA virus
This study visualizes the interior of a dsRNA virus using cryo-electron microscopy, revealing the organization of the genome of cytoplasmic polyhedrosis virus together with its transcriptional enzyme complex in both quiescent and transcribing states.
- Xing Zhang
- , Ke Ding
- & Z. Hong Zhou
-
Article |
 Gating machinery of InsP3R channels revealed by electron cryomicroscopy
Gating machinery of InsP3R channels revealed by electron cryomicroscopy
This study has determined the electron cryomicroscopy structure of the mammalian type 1 InsP3 receptor in a ligand-free state at 4.7 Å resolution; although the central Ca2+-conduction pathway is similar to other ion channels, the unique architecture of the C-terminal domains of the tetrameric channel suggests that a distinctive allosteric mechanism underlies the activation of InsP3 gating.
- Guizhen Fan
- , Matthew L. Baker
- & Irina I. Serysheva
-
Article |
 Cryo-electron microscopy structure of the Slo2.2 Na+-activated K+ channel
Cryo-electron microscopy structure of the Slo2.2 Na+-activated K+ channel
The structure of the full-length Slo2.2 Na+-activated K+ channel is determined by cryo-electron microscopy, revealing features that explain the high conductance and gating mechanism of the Slo K+ channel family.
- Richard K. Hite
- , Peng Yuan
- & Roderick MacKinnon
-
Article |
 Architecture of the mammalian mechanosensitive Piezo1 channel
Architecture of the mammalian mechanosensitive Piezo1 channel
Piezo1, a mechanosensitive cation channel, senses shear stress of blood flow for proper blood vessel development, regulates red blood cell function and controls cell migration and differentiation; here a trimeric architecture of this novel class of ion channel is reported, suggesting that Piezo1 may use its peripheral propeller-like ‘blades’ as force sensors to gate the central ion-conducting pore.
- Jingpeng Ge
- , Wanqiu Li
- & Maojun Yang
-
Article |
 Structure of mammalian eIF3 in the context of the 43S preinitiation complex
Structure of mammalian eIF3 in the context of the 43S preinitiation complex
The cryo-electron microscopy structure of the eukaryotic initiation factor 3 (eIF3) within the larger 43S complex is determined; the improved resolution enables visualization of the secondary structures of the subunits, as well as the contacts between eIF3 and both eIF2 and DHX29.
- Amedee des Georges
- , Vidya Dhote
- & Yaser Hashem
-
Article |
 An atomic structure of human γ-secretase
An atomic structure of human γ-secretase
The atomic structure of human γ-secretase at 3.4 Å resolution, determined by single-particle cryo-electron microscopy.
- Xiao-chen Bai
- , Chuangye Yan
- & Yigong Shi
-
Letter |
 Structural basis for stop codon recognition in eukaryotes
Structural basis for stop codon recognition in eukaryotes
All eukaryotes utilize a single termination factor, eRF1, to halt translation when the ribosome encounters one of three possible stop codons; here electron cryo-microscopy structures of ribosome–eRF1 complexes in the process of recognizing each stop codon reveal how stop codons are discriminated from sense codons.
- Alan Brown
- , Sichen Shao
- & V. Ramakrishnan
-
Article |
 Structure of the eukaryotic MCM complex at 3.8 Å
Structure of the eukaryotic MCM complex at 3.8 Å
Cryo-electron microscopy is used to visualize the double hexamer of the eukaryotic minichromosome maintenance complex (MCM), which is assembled during the G1 phase of DNA replication; two interdigitated hexamers have a central channel that tightly fits a DNA duplex, and the orientation of the tilted single hexamers sheds light on many functional aspects, particularly in the initial origin DNA melting.
- Ningning Li
- , Yuanliang Zhai
- & Ning Gao
-
Article |
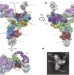 The architecture of the spliceosomal U4/U6.U5 tri-snRNP
The architecture of the spliceosomal U4/U6.U5 tri-snRNP
This study determines the structure of the spliceosomal tri-snRNP complex (containing three small nuclear RNAs and more than 30 proteins) by single-particle cryo-electron microscopy; the resolution is sufficient to discern the organization of RNA and protein components involved in spliceosome activation, exon alignment and catalysis.
- Thi Hoang Duong Nguyen
- , Wojciech P. Galej
- & Kiyoshi Nagai
-
Article |
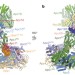 Atomic structure of the APC/C and its mechanism of protein ubiquitination
Atomic structure of the APC/C and its mechanism of protein ubiquitination
A cryo-electron microscopy determination of the atomic structures of anaphase-promoting complex (APC/C)–coactivator complexes with either Emi1 or a UbcH10–ubiquitin conjugate.
- Leifu Chang
- , Ziguo Zhang
- & David Barford
-
Letter |
 Structural basis for retroviral integration into nucleosomes
Structural basis for retroviral integration into nucleosomes
Retroviruses such as HIV rely on the intasome, a tetramer of integrase protein bound to the viral DNA ends interacting with host chromatin, for integration into the host genome; the structure of the intasome as it interacts with a nucleosome is now solved, giving insight into the integration process.
- Daniel P. Maskell
- , Ludovic Renault
- & Peter Cherepanov
-
Letter |
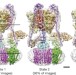 Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase
Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase
Electron cryomicroscopy shows structures of three distinct rotational states of the V-ATPase from Saccharomyces cerevisiae.
- Jianhua Zhao
- , Samir Benlekbir
- & John L. Rubinstein
-
Letter |
 Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles
Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles
Structures of actin-like ParM filaments at near-atomic resolution and their arrangements into doublets reveal how subunits and filaments come together to segregate low-copy-number plasmid R1 in Escherichia coli, producing the simplest known mitotic machinery.
- Tanmay A. M. Bharat
- , Garib N. Murshudov
- & Jan Löwe
-
Article |
 Structure of the human 80S ribosome
Structure of the human 80S ribosome
The structure of the human ribosome at high resolution has been solved; by combining single-particle cryo-EM and atomic model building, local resolution of 2.9 Å was achieved within the most stable areas of the structure.
- Heena Khatter
- , Alexander G. Myasnikov
- & Bruno P. Klaholz
-
Letter |
 Atomic structure of anthrax protective antigen pore elucidates toxin translocation
Atomic structure of anthrax protective antigen pore elucidates toxin translocation
Cryo-electron microscopy determination of anthrax toxin protective antigen pore structure at a resolution of 2.9 Å, revealing the catalytic Φ-clamp and the membrane-spanning translocation channel.
- Jiansen Jiang
- , Bradley L. Pentelute
- & Z. Hong Zhou
-
Letter |
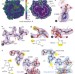 Structure of the E. coli ribosome–EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM
Structure of the E. coli ribosome–EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM
A single particle cryo-EM structure of the 70S ribosome in complex with the elongation factor Tu breaks the 3 Å resolution barrier of the technique and locally exceeds the resolution of previous crystallographic studies, revealing all modifications in rRNA and explaining their roles in ribosome function and antibiotic binding.
- Niels Fischer
- , Piotr Neumann
- & Holger Stark
-
Letter |
 Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase
Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase
Electron cryomicroscopy of a complete mitochondrial ATP-synthase dimer reveals the elusive structure of the essential a-subunit.
- Matteo Allegretti
- , Niklas Klusch
- & Karen M. Davies
-
Article |
 Mechanistic insights into the recycling machine of the SNARE complex
Mechanistic insights into the recycling machine of the SNARE complex
Using single-particle electron cryomicroscopy, several structures are reported which illuminate the mechanisms of action of the ATPase NSF that disassembles the SNARE complex into individual protein components.
- Minglei Zhao
- , Shenping Wu
- & Axel T. Brunger
-
Article |
 Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution
Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution
Using electron cryomicroscopy, the structure of the closed-state rabbit ryanodine receptor RyR1 in complex with its modulator FKBP12 is solved at 3.8 Å; in addition to determining structural details of the ion-conducting channel domain, three previously uncharacterized domains help to reveal a molecular scaffold that allows long-range allosteric regulation of channel activities.
- Zhen Yan
- , Xiao-chen Bai
- & Nieng Yan
-
Article |
 Structure of a mammalian ryanodine receptor
Structure of a mammalian ryanodine receptor
Using electron cryomicroscopy, the closed-state structure of rabbit RyR1 is determined at 4.8 Å resolution; analysis confirms that the RyR1 architecture consists of a six-transmembrane ion channel with a cytosolic α-solenoid scaffold, and suggests a mechanism for Ca2+-induced channel opening.
- Ran Zalk
- , Oliver B. Clarke
- & Andrew R. Marks
-
Letter |
 Structure of the F-actin–tropomyosin complex
Structure of the F-actin–tropomyosin complex
Electron cryomicroscopy reveals the three-dimensional structure of F-actin at a resolution of 3.7 Å in complex with tropomyosin at a resolution of 6.5 Å; the stabilizing interactions and the effects of disease-causing mutants are also investigated.
- Julian von der Ecken
- , Mirco Müller
- & Stefan Raunser
-
Article |
 Architecture and conformational switch mechanism of the ryanodine receptor
Architecture and conformational switch mechanism of the ryanodine receptor
Using electron cryomicroscopy, the structure of the rabbit RyR1 calcium channel is determined at 6.1 Å resolution in the closed state and 8.5 Å in the open state, revealing how calcium binding to the EF-hand of RyR1 regulates channel opening and facilitates calcium-induced calcium release.
- Rouslan G. Efremov
- , Alexander Leitner
- & Stefan Raunser
-
Letter |
 Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter
Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter
The subnanometre-resolution electron cryomicroscopy structure of TmrAB, a heterodimeric ABC transport protein, in a nucleotide-free, inward-facing conformation, is determined.
- JungMin Kim
- , Shenping Wu
- & Yifan Cheng
-
Letter |
 The complete structure of the large subunit of the mammalian mitochondrial ribosome
The complete structure of the large subunit of the mammalian mitochondrial ribosome
The structure of the 39S large mitoribosome subunit is solved by cryo-electron microscopy at an impressive 3.4 Å resolution, revealing the location of 50 ribosomal proteins, the peptidyl transferase centre, the tRNAs within this active site, and the nascent peptide chain within the exit tunnel.
- Basil J. Greber
- , Daniel Boehringer
- & Nenad Ban
-
Article |
 Architecture of mammalian respiratory complex I
Architecture of mammalian respiratory complex I
Complex I is the first enzyme of the mitochondrial electron transport chain and it is essential for oxidative phosphorylation in mammalian mitochondria; here the electron cryo-microscopy structure of complex I from bovine heart mitochondria is reported, advancing knowledge of its structure in mammals.
- Kutti R. Vinothkumar
- , Jiapeng Zhu
- & Judy Hirst
-
Article |
 Molecular architecture and mechanism of the anaphase-promoting complex
Molecular architecture and mechanism of the anaphase-promoting complex
The anaphase-promoting complex/cyclosome (APC/C) is a large E3 ligase that mediates ubiquitin-dependent proteolysis of cell cycle regulatory proteins; here the complete secondary structure architecture of human APC/C complexed with its coactivator CDH1 and substrate HSL1 is determined at 7.4 Å resolution, revealing allosteric changes induced by the coactivator that enhance affinity for UBCH10–ubiqutin.
- Leifu Chang
- , Ziguo Zhang
- & David Barford
-
Article |
 Three-dimensional structure of human γ-secretase
Three-dimensional structure of human γ-secretase
The three-dimensional structure of intact human γ-secretase complex at 4.5 Å resolution is revealed by cryo-electron-microscopy single-particle analysis; the complex comprises a horseshoe-shaped transmembrane domain containing 19 transmembrane segments, and a large extracellular domain from nicastrin, which sits immediately above the hollow space formed by the horseshoe.
- Peilong Lu
- , Xiao-chen Bai
- & Yigong Shi
-
Letter |
 Structural rearrangements of a polyketide synthase module during its catalytic cycle
Structural rearrangements of a polyketide synthase module during its catalytic cycle
Polyketide synthases (PKSs) are multidomain enzymes that produce polyketides, which form the basis of many therapeutic agents; here, electron cryo-microscopy is used to probe the structure of an intact module of a multi-enzyme PKS in different functional states.
- Jonathan R. Whicher
- , Somnath Dutta
- & Georgios Skiniotis
-
Article |
 Structure of a modular polyketide synthase
Structure of a modular polyketide synthase
Polyketide synthases are multidomain enzymes that produce polyketides, which form the basis of many therapeutic agents; here, electron cryo-microscopy is used to establish the structure of a bacterial full-length module, and to elucidate the structural basis of both intramodule and intermodule substrate transfer.
- Somnath Dutta
- , Jonathan R. Whicher
- & Georgios Skiniotis
-
Letter |
 Structure of the AcrAB–TolC multidrug efflux pump
Structure of the AcrAB–TolC multidrug efflux pump
Many bacteria are able to survive in the presence of antibiotics in part because they possess pumps that can remove a broad range of small molecules; here, the structure of one such pump, AcrAB–TolC, is determined using X-ray crystallography and cryo-electron microscopy.
- Dijun Du
- , Zhao Wang
- & Ben F. Luisi
-
Article |
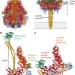 Mechanism of Tc toxin action revealed in molecular detail
Mechanism of Tc toxin action revealed in molecular detail
High-resolution structures of the Photorhabdus luminescens TcA toxin subunit and the entire Tc toxin complex reveal important new insights into Tc complex structure and function.
- Dominic Meusch
- , Christos Gatsogiannis
- & Stefan Raunser
-
Letter |
 Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion
Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion
Nascent secretory and membrane proteins are targeted to the Sec61 protein-conducting channel for translocation across or insertion into the endoplasmic reticulum membrane; here cryo-electron microscopy structures of eukaryotic ribosome–channel complexes show how this channel opens vertically during translocation of a secretory protein into the lumen of the endoplasmic reticulum and how it opens laterally during insertion of a transmembrane domain into the lipid bilayer.
- Marko Gogala
- , Thomas Becker
- & Roland Beckmann
-
Article |
 Architecture of the large subunit of the mammalian mitochondrial ribosome
Architecture of the large subunit of the mammalian mitochondrial ribosome
Cryo-electron microscopy combined with chemical crosslinking and mass spectrometry is used to determine the structure of the large subunit of the mammalian mitoribosome; this structure provides detailed structural insight, particularly of the molecular architecture of the polypeptide exit site, which has been structurally remodelled during evolution, presumably to help facilitate the membrane insertion of the highly hydrophobic proteins encoded by the mitochondrial genome.
- Basil J. Greber
- , Daniel Boehringer
- & Nenad Ban
-
Article |
 TRPV1 structures in distinct conformations reveal activation mechanisms
TRPV1 structures in distinct conformations reveal activation mechanisms
Using a peptide toxin and small vanilloid agonists as pharmacological probes, high-resolution electron cryo-microscopy structures of rat TRPV1–ligand complexes are solved; these structures highlight conformational differences between TRP and voltage-gated ion channels in their active states, and suggest a dual gating mechanism that may account for the ability of members of the TRP channel superfamily to integrate diverse physiological signals.
- Erhu Cao
- , Maofu Liao
- & David Julius
-
Article |
 Structure of the TRPV1 ion channel determined by electron cryo-microscopy
Structure of the TRPV1 ion channel determined by electron cryo-microscopy
A high-resolution electron cryo-microscopy structure of the rat transient receptor potential (TRP) channel TRPV1 in its ‘closed’ state is presented; the overall structure of this ion channel is found to share some common features with voltage-gated ion channels, although several unique, TRP-specific features are also characterized.
- Maofu Liao
- , Erhu Cao
- & Yifan Cheng
-
Letter |
 Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit
Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit
A sub-nanometre reconstruction of a 40S complex containing eIF3 and a hepatitis C virus (HCV)-like internal ribosome entry site (IRES) shows that the IRES displaces eIF3 from the 40S and sequesters it to gain access to the 40S subunit.
- Yaser Hashem
- , Amedee des Georges
- & Joachim Frank
-
Letter |
 Structure of the SecY channel during initiation of protein translocation
Structure of the SecY channel during initiation of protein translocation
Newly synthesized proteins are targeted to the SecY protein-conducting channel for translocation across the membrane; here, cryo-electron microscopy structures of inactive and active ribosome–channel complexes are presented, revealing that ribosome binding does not result in major structural changes to transmembrane regions of the channel, and that stable channel opening requires loop insertion of the translocating nascent chain.
- Eunyong Park
- , Jean-François Ménétret
- & Christopher W. Akey
-
Letter |
 Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics
Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics
The structure of the HIV-1 capsid is analysed by cryo-electron microscopy and cryo-electron tomography, allowing presentation of an all-atom molecular dynamics model of the entire capsid.
- Gongpu Zhao
- , Juan R. Perilla
- & Peijun Zhang
-
Article |
 Structures of the human and Drosophila 80S ribosome
Structures of the human and Drosophila 80S ribosome
High-resolution cryo-EM density maps are used to present the structures of Drosophila and human 80S ribosomes in complex with eEF2, E-site transfer RNA and Stm1-like proteins, and reveal the presence of two additional structural layers in the ribosomes of metazoan eukaryotes.
- Andreas M. Anger
- , Jean-Paul Armache
- & Roland Beckmann
-
Letter |
 A syringe-like injection mechanism in Photorhabdus luminescens toxins
A syringe-like injection mechanism in Photorhabdus luminescens toxins
The TcA component of Photorhabdus luminescens ABC-type toxin complexes forms a transmembrane pore and injects TcC, the functional component of the toxin, into the target cell by means of a syringe-like mechanism.
- Christos Gatsogiannis
- , Alexander E. Lang
- & Stefan Raunser
-
Article |
 Structural visualization of key steps in human transcription initiation
Structural visualization of key steps in human transcription initiation
Cryo-electron microscopy structures of key intermediates during the sequential assembly of the pre-initiation complex are presented; structures of the closed and open-promoter complexes allow insights into the process of promoter melting.
- Yuan He
- , Jie Fang
- & Eva Nogales
-
Letter |
 The architecture of human general transcription factor TFIID core complex
The architecture of human general transcription factor TFIID core complex
The structures of three distinct human transcription factor IID (TFIID) protein assemblies are solved using cryo-electron microscopy; by incorporating TAF8 and TAF10, the key structural changes that remodel TFIID during assembly are determined, particularly the transition from a symmetric core-TFIID to an asymmetric holo-complex.
- Christoph Bieniossek
- , Gabor Papai
- & Imre Berger
-
Letter |
 Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy
Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy
A hybrid cryo-electron microscopy/tomography approach is used to solve the structure of the immature Mason–Pfizer monkey virus Gag lattice at a resolution of 8 Å, allowing the derivation of a model for the structure of retroviral capsid in the immature Gag shell.
- Tanmay A. M. Bharat
- , Norman E. Davey
- & John A. G. Briggs
-
Letter |
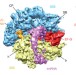 The complex of tmRNA–SmpB and EF-G on translocating ribosomes
The complex of tmRNA–SmpB and EF-G on translocating ribosomes
Stalled bacterial ribosomes can be rescued by interaction with SmpB protein and a highly structured transfer-messenger RNA, and a cryo-electron microscopy map of this complex now shows how EF-G-dependent translocation of this non-canonical ligand is facilitated by conformational changes in the ribosome and the transfer-messenger RNA.
- David J. F. Ramrath
- , Hiroshi Yamamoto
- & Christian M. T. Spahn
-
Article |
 Structure and mechanism of the chromatin remodelling factor ISW1a
Structure and mechanism of the chromatin remodelling factor ISW1a
- Kazuhiro Yamada
- , Timothy D. Frouws
- & Timothy J. Richmond
-
Letter |
 Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
During translation, tRNAs enter the ribosome and then move sequentially through three sites, known as A, P and E, as they transfer their attached amino acids onto the growing peptide chain. How the ribosome facilitates tRNA translocation between the sites remains largely unknown. Now a study uses multiparticle cryoelectron microscopy of a ribosome bound to the translation elongation factor, EF-G, to get information about tRNA movement. It identifies two new substates and sees that translocation is linked to unratcheting of the 30S ribosomal subunit.
- Andreas H. Ratje
- , Justus Loerke
- & Christian M. T. Spahn
-
Article |
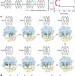 Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
During protein synthesis within the ribosome, transfer RNAs (tRNAs) move sequentially through different sites as their attached amino acids are transferred onto the growing protein chain. Large conformational movements accompany this process. Here, a staggering 1.9 million electron cryomicroscopy images of the ribosome have been processed to visualize these changes. The results reveal that the ribosome functions as a Brownian machine that couples spontaneous changes driven by thermal energy to directed movement.
- Niels Fischer
- , Andrey L. Konevega
- & Holger Stark
-
News & Views |
 Translocation in slow motion
Translocation in slow motion
Time-resolved electron microscopy can capture structural changes in active macromolecular complexes, but detailed imaging is essential. The dynamics of one step in protein synthesis has been deduced from two million images.
- Måns Ehrenberg

 Structure of transcribing mammalian RNA polymerase II
Structure of transcribing mammalian RNA polymerase II