« Prev Next »
Birds do it, and bees do it. Indeed, researchers estimate that over 99.99% of eukaryotes do it, meaning that these organisms reproduce sexually, at least on occasion. But why is sexual reproduction so commonplace?
People typically employ several arguments in their efforts to explain the prevalence of sexual reproduction. One such argument is that organisms engage in sex because it is pleasurable. However, from an evolutionary perspective, this explanation arrived only moments ago. The first eukaryotes to engage in sex were single-celled protists that appeared approximately 2 billion years ago, over 1.3 billion years before development of the first animals with neurons capable of assessing pleasure. These bacteria (as well as their modern counterparts) engaged in genetic exchange via processes such as conjugation, transformation, and transduction, all of which fall under the umbrella of parasexuality. Surely, pleasure was not in a bacterium's realm of experience.
A second, more serious argument is that sex generates variable offspring upon which natural selection can act. This is one of the oldest explanations for sexual reproduction, tracing back to the work of German biologist August Weismann in the late 1800s. Although this explanation may very well account for why sexual reproduction is so commonplace, the explanation is far more subtle than many people realize for two reasons. First, sex does not always increase the variability among offspring. Second, producing more variable offspring is not necessarily favorable. In the next two sections, we describe these flaws in Weismann's explanation for sex, so that we can better understand the processes that help and those that hinder the evolution of sex.
The Importance of Sexual Reproduction
To develop a better understanding of why sexual reproduction is so commonplace, it is helpful to start with an examination of some of the most common erroneous beliefs regarding the relationship between sex and natural selection, including those described in the following sections.
Sex Does Not Always Generate More Variable Offspring
Many people assume that sexual reproduction is critical to evolution because it always results in the production of genetically varied offspring. In truth, however, sex does not always increase variation. Imagine, for instance, the simple case of a single gene that contributes to height in a diploid organism; here, individuals with genotype aa are shortest, those with genotype Aa are of intermediate height, and those with genotype AA are tallest (Figure 1). Now, for the sake of argument, imagine that the shortest individuals can hide safely, the tallest individuals are too big to be eaten by predators, and the intermediate-height individuals are heavily preyed upon. Among those lucky few organisms who survive to reproduce, there will be a great deal of variation in height, with plenty of tall individuals and plenty of short individuals. What would sex accomplish in this case? Here, mating would bring the population back to Hardy-Weinberg proportions, producing fewer offspring at the extremes of height and more offspring in the middle. That is, sex would reduce variation in height, relative to a population that reproduces asexually.
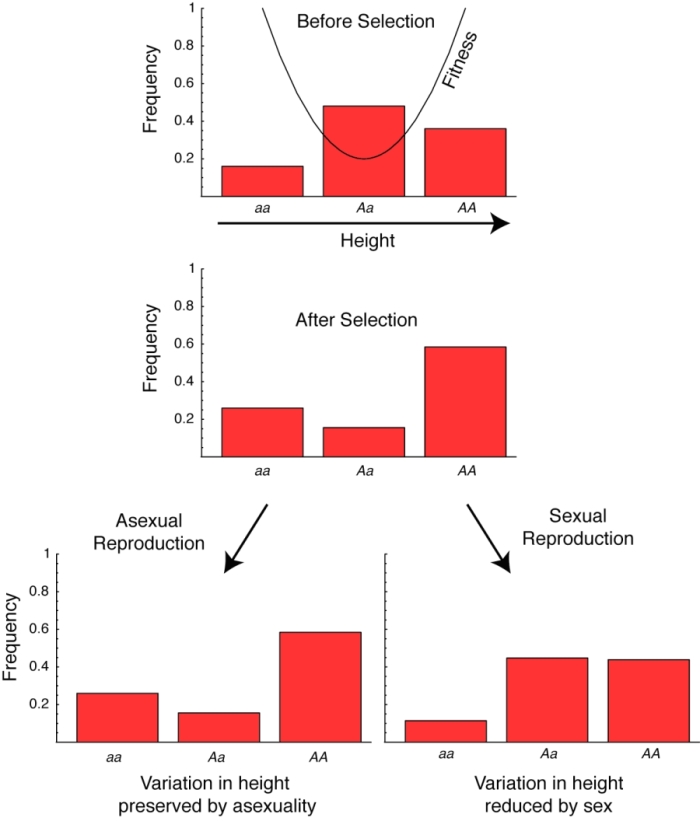
This example is overly simplified, but it serves to illustrate a general point: Selection can build more variation than one would expect in a population in which genes are well mixed. In such cases, sex reduces variation by mixing together genes from different parents. This problem arises in the case of a single gene whenever heterozygotes are less fit, on average, than homozygotes. (In this case, the heterozygote need not have the lowest fitness; rather, its fitness must only be close to that of the least-fit homozygote.) The problem also arises in more complicated cases involving multiple genes whenever those genes interact in such a way that intermediate genotypes (say, + - + - + -) have lower fitness than the average of the extreme genotypes (here, + + + + + + and - - - - - -).
In general, mathematical models have confirmed that selection builds more variation than expected from randomly combined genes whenever fitness surfaces are positively curved, with intermediate genotypes having lower-than-expected fitness. In such cases, sexual reproduction and recombination destroy the genetic associations that selection has built and therefore result in decreased (rather than increased) variation among offspring. The term "epistasis" is used to describe such gene interactions, and cases in which the intermediate genotypes are less fit than expected (based on the fitness of the more extreme genotypes) are said to exhibit "positive epistasis." (As a technical aside, the curvature of the fitness surface should be measured on a multiplicative scale, so that if the fitness of + + + + + + individuals was 2 and the fitness of - - - - - -individuals was 1, then the fitness of + - + - + - individuals could be expected to be √2×1 = 1.41.)
Producing Variable Offspring Can Hinder the Evolution of Sex
Interestingly, even when sex does restore genetic variation, producing more variable offspring does not necessarily promote the evolution of sex. Again, this reality refutes one of the arguments often raised in the attempt to explain the relationship between sex and evolution. To understand how this operates, consider another simple case involving a single gene, but this time, assume that heterozygotes (rather than homozygotes) are fittest. The gene responsible for sickle-cell anemia provides a great real-life example. Here, people who are heterozygous for the sickle-cell allele (genotype Ss) are less susceptible to malarial infection yet have a sufficient number of healthy red blood cells; on the other hand, SS homozygotes are more susceptible to malaria, while ss homozygotes are more susceptible to anemia. Thus, in areas infested with the protozoans that cause malaria, adults who have survived to reproduce are more likely to have the Ss genotype than would be expected based on Hardy-Weinberg proportions. In such populations in which heterozygotes are in excess, sexual reproduction regenerates homozygotes from crosses among heterozygotes. Although this indeed results in greater genetic variation among offspring, the variation consists largely of homozygotes with low fitness.
Yet again, this simple example illustrates a more general point: Parents that have survived to reproduce tend to have genomes that are fairly well adapted to their environments. Mixing two genomes through sex and genetic recombination tends to produce offspring that are less fit, simply because a mixture of genes from both parents has no guarantee of functioning as well as the parents' original gene sets. In fact, mathematical models have confirmed that when selection builds associations among genes, destroying these associations through sex and recombination tends to reduce offspring fitness. This reduction in fitness caused by sex and recombination is referred to as the "recombination load" (or the "segregation load" when referring specifically to segregation at a single diploid gene).
The reason that the recombination load is a problem for the evolution of sex is better appreciated by looking at evolution at the level of the gene. Imagine a gene that promotes sexual reproduction, such as by making it more likely that a plant will reproduce via sexually produced seeds as opposed to some asexual process (e.g., budding, asexual seeds, etc.). Carriers of this gene will tend to produce less fit offspring because sexual reproduction and recombination break apart the genetic associations that have been built by past selection. The gene promoting sex will fail to spread if the offspring die at too high a high rate, even if the offspring are more variable. Indeed, theoretical models developed in the 1980s and 1990s demonstrate that genes promoting sex and recombination increase in frequency only when all of the following conditions hold true:
- The population is under directional selection. (This means that increased variation can improve the response to selection.)
- Fitness surfaces are negatively curved. (This means that sex and recombination can restore variation eliminated by past selection.)
- The surface curvature is not too strong. (If too strong, the recombination load is severe).
Unfortunately, empirical data have not indicated that fitness surfaces curve in just the right way for these models to work in real-life situations.
Sex Can Be Too Costly to Evolve
To make matters worse, sexual reproduction often entails costs beyond the recombination load described earlier. To reproduce sexually, an individual must take the time and energy to switch from mitosis to meiosis (this step is especially relevant in single-celled organisms); it must find a willing mate; and it must risk contracting sexually transmitted diseases. Moreover, an individual that reproduces sexually passes only half of its genes to its offspring, whereas it would have transmitted 100% of its genes to progeny that were produced asexually. (This last cost is often called the "twofold cost of sex.") Thus, unless the individual's sexual partner contributes enough resources to double the number of offspring, an organism that reproduces sexually passes on fewer copies of its genes than an organism that reproduces asexually.
These are substantial costs—so substantial that many species have evolved mechanisms to ensure that sex occurs only when it is least costly. For instance, organisms including aphids and daphnia reproduce asexually when resources are abundant and switch to sex only at the end of the season, when the potential for asexual reproduction is limited and when potential mates are more available. Similarly, many single-celled organisms have sex only when starved, which minimizes the time cost of switching to meiosis because mitotic growth has already ceased.
Although various mechanisms might reduce the costs of sex, it is still commonly assumed that sex is more costly than asexual reproduction, raising yet another obstacle for the evolution of sex.
Why, Then, Is Sexual Reproduction So Common?
The aforementioned points might lead one to conclude that sex is a losing enterprise. However, sex is incredibly common. Furthermore, even though asexual lineages do arise, they rarely persist for long periods of evolutionary time. Among flowering plants, for example, predominantly asexual lineages have arisen over 300 times, yet none of these lineages is very old. Furthermore, many species can reproduce both sexually and asexually, without the frequency of asexuality increasing and eliminating sexual reproduction altogether. What, then, prevents the spread of asexual reproduction?
The first generation of mathematical models examining the evolution of sex made several simplifying assumptions—namely, that selection is constant over time and space, that all individuals engage in sex at the same rate, and that populations are infinitely large. With such simplifying assumptions, selection remains the main evolutionary force at work, and sex and recombination serve mainly to break down the genetic associations built up by selection. So, it is perhaps no wonder that this early generation of models concluded that sex would evolve only under very restrictive conditions.
Subsequent models have relaxed these assumptions in a number of ways, attempting to better capture many of the complexities involved in real-world evolution. The results of these second-generation models are briefly summarized in the following sections.
Sex Evolves When Selection Changes Over Time
Current models indicate that sex evolves more readily when a species' environment changes rapidly. When the genetic associations built up by past selection are no longer favorable, sex and recombination can improve the fitness of offspring, thereby turning the recombination load into an advantage. One important source of environmental change is a shift in the community of interacting species, especially host and parasite species. This is the so-called "Red Queen" hypothesis for the evolution of sex, which refers to the need for a species to evolve as fast as it can just to keep apace of coevolving species. (The name of this hypothesis comes from Lewis Carroll's Through the Looking Glass, in which Alice must run as fast as she can "just to stay in place.") Increased allocation to sexual reproduction can evolve because of "Red Queen" interactions, but only if selection is strong enough to cause rapid switches in which gene combinations are favorable.
Sex Evolves When Selection Changes Over Space
Sex can also be favored when selection varies over space, as long as the genetic associations created by migration are locally disadvantageous. Whether this requirement is common in nature remains an open question.
Sex Evolves When Organisms Are Less Adapted to Their Environment
Organisms that reproduce both sexually and asexually tend to switch to sex under stressful conditions. Mathematical models have revealed that it is much easier for sex to evolve if individuals that are adapted to their environment reproduce asexually and less fit individuals reproduce sexually. In this way, well-adapted genotypes are not broken apart by recombination, but poorly adapted genotypes can be recombined to create new combinations in offspring.
Sex Evolves When Populations Are Finite
Models that account for the fact that population sizes are finite have found that sex and recombination evolve much more readily. With a limited number of individuals in a population, selection erodes easily accessible variation, leaving only hidden variation (Figure 2). Recombination can then reveal this hidden variation, improving the response to selection. By improving the response to selection, genes that increase the frequency of sex become associated with fitter genotypes, which rise in frequency alongside them. Interestingly, the requirement that fitness surfaces exhibit weak and negative curvature is relaxed in populations of finite size; here, fitness surfaces may be uncurved or positively curved and still favor sex.
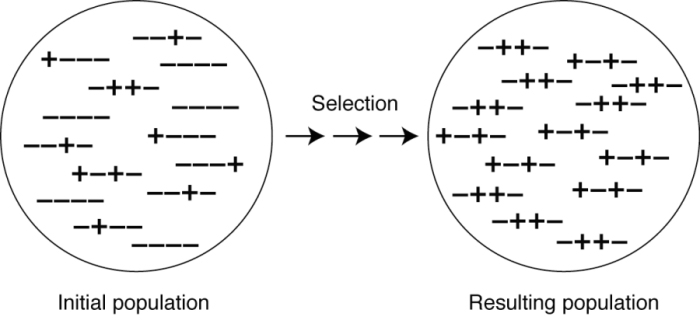
This last result is particularly interesting, because it suggests that August Weismann might have been right all along in arguing that sex evolved to generate variation. Modeling Weismann's hypothesis with infinitely large populations failed because variation is too easily generated by mutation and too easily maintained by selection within these populations. Altering this size-related assumption by modeling selection among a finite number of individuals reveals just how important sex and recombination are as processes that allow genes residing in different individuals to be brought together, thereby producing new genotypic combinations upon which selection can act.
References and Recommended Reading
De Visser, J. A. G. M., & Elena, S. F. The evolution of sex: Empirical insights into the roles of epistasis and drift. Nature Reviews Genetics 8, 139–149 (2007) doi:10.1038/nrg1985 (link to article)
Felsenstein, J. The evolutionary advantage of recombination. Genetics 78, 737–756 (1974)
Otto, S. P., & Lenormand, T. Resolving the paradox of sex and recombination. Nature Reviews Genetics 3, 252–261 (2002) (link to article)






























