« Prev Next »
Plenty of news articles and advertisements all promote the beneficial effects of omega-3 and omega-6 fatty acids. We know that fats provide caloric energy in our diet, but why should the particular kind of fat we eat make any difference? Specific fatty acids are the starting material for many vital signal molecules in plants and animals. Mammals cannot synthesize these fatty acid precursor molecules on their own, so a failure to obtain these fats from the diet can have major negative consequences. How were these molecules discovered and what specific features of their chemistry make them necessary? A fat-free diet for rats, hormonal secretions from the prostate gland, aspirin, and innovative chemical syntheses, are all parts of this developing story that help us explain the biological importance of these molecules.
What Happens When Fat is Removed from the Diet?
By the early twentieth century, scientists and doctors widely agreed on the basic set of vitamins needed for human health. But what about other nutrients? Working at the University of Minnesota, Burr & Burr (1929) examined the effect of fat-free diets on rats. They fed rats a diet containing sufficient calories, with protein and all of the known vitamins, but without fat. The control group of rats was fed the same diet, but containing fat. They carefully observed the health of the rats over several months. Over that time, the rats on the fat-free diet failed to thrive, developed severe skin and kidney problems, and often died within weeks. Interestingly, they could return the sickly animals to good health by adding some dietary fats, or small amounts of liver, back to their food, but many other fats had no beneficial effect. Why were these animals sick? Burr & Burr proposed several hypotheses. Perhaps the strain on the animals caused by synthesizing all of their own fats was the cause. Could there be a new fat-soluble vitamin (vitamin F), which was needed for animal health? Or were there specific features of the fat itself that were necessary? They knew that there are several kinds of fatty acids in the human diet, so they decided to answer some of these questions by systematically feeding the sick animals a selection of known fats to see if any of them had an effect (Figure 1). They eventually discovered that adding back two particular purified fatty acids, linolenic and linoleic acids, restored the sick animals to health, while the other fatty acids were unable to do so (Burr & Burr 1932). This led to the recognitions of linolenic and linoleic acids as essential fatty acids.
Linolenic and Linoleic Acids: The Special Fats
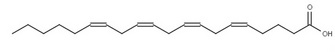
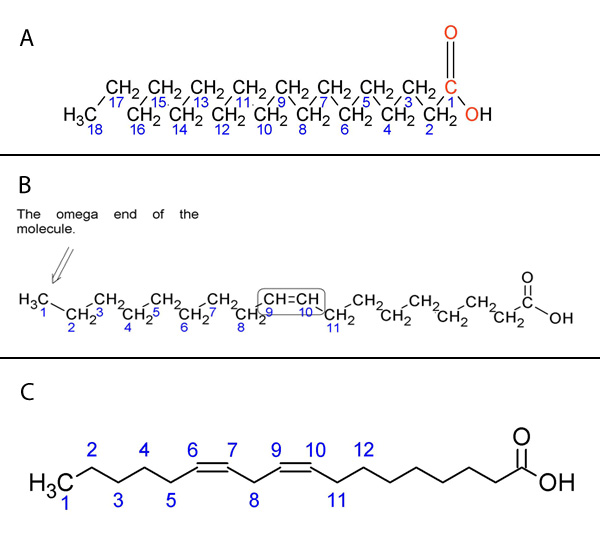
The position of these double bonds can be described in several ways. The most common is to begin numbering the carbons from the carboxylic acid functional group, with that carbon labeled as carbon 1. Following this system, linoleic acid has double bonds at the 9 and 12 positions, whereas linolenic acid has double bonds at the 9, 12, and 15 positions. An alternate numbering system using Greek letters is widely used to label the carbons starting from the carbon farthest from the functional group — which is defined as the ω (omega) 1 carbon. If we examine linolenic acid, we see that the carbon-carbon double bond furthest from the carboxylic acid functional group is on the third carbon from the omega end, so linolenic acid is an omega-3 fatty acid. Similarly, linoleic acid is an omega-6 fatty acid.
What Does the Body Do with the Fats We Consume?
Through meticulous physiological experiments, scientists have tracked the metabolism of radioactively-labeled fatty acids, and discovered that we have enzymes that can alter the structure of dietary fatty acids as needed. These enzymes can add carbons, to increase the length of the fatty acid chain, or insert or delete carbon-carbon double bonds. These conversions happen in the liver, brain and retina in our body. However, we are limited by the enzymes we produce.
Some of these essential fatty acids, obtained in our diet, are converted into a 20-carbon fatty acid containing 4 carbon-carbon double bonds. This omega-6 fatty acid, arachidonic acid, is an important starting material for three different types of signaling molecules (Figure 3). Similar research found pathways that converted fat from our diet into the 22 carbon omega-3 fatty acid, docosahexaenoic acid (DHA). Identifying these different contributing signaling molecules ultimately required work in multiple countries using a variety of experimental techniques.
How Did Scientists Determine a Function for Fatty Acids?
In the 1930s, scientists in the United States and England indicated that human semen contained biologically active material that could lead to uterine contractions, reduce blood pressure, and stimulate smooth muscle. Similarly, Ulf Svante von Euler found a comparable substance in the prostate glands secretions of several mammals. When examined in more detail, scientists found that this crude material was fat soluble, and acidic, and named it prostaglandin. Was this active substance one molecule or a mixture of several molecules? Could it be purified enough so that scientists could determine the exact molecular structure, and perhaps create stable forms useful in treating disease?

When the Swedish scientists studied the structure of these purified molecules, they found that that these molecules all contained double bonds located in roughly the same positions as the double bonds in the essential polyunsaturated fatty acids (Figure 4). Was it possible that the essential fatty acids were the precursors to these potent signaling molecules? The Swedish group contacted van Dorp in Holland, who had radioactively labeled linoleic acid, and requested a sample of this material to add to their sheep gland extracts. Unknown to them, van Dorp was planning on doing the same experiment, but graciously shared this material with the Swedish group. In 1964 the two groups separately, but simultaneously, conducted the experiment, and both groups found the same result. When tissues that could produce prostaglandins were treated with radioactively labeled linoleic acid made with radioactive carbon, they found that the radioactive carbon atoms appeared in the prostaglandins. What was the interpretation of this observation? The fact that radioactive carbons initially present in the linoleic acid were later showing up in the produced prostaglandins indicated that fatty acids were indeed the precursor molecules to prostaglandins.
What Function Do Prostaglandins Play in the Body?
At this point these two separate lines of research merged, with the realization that the essential fatty acids earlier recognized by Burr and others as key to animal health were the starting molecules used to produce the potent prostaglandins. This later work was extremely challenging in two respects. First, the prostaglandins were extremely potent in small quantities, with fractions of a milligram capable of changing human circulation. Second, they were also very unstable and inactivated rapidly in biological systems. It was therefore very difficult to store prostaglandins for long periods of time without them degrading into unusable forms.
Innovative work in organic chemistry, both in defining the structures of molecules using very small amounts, and by synthesizing the proposed structures for these molecules in the lab, were important tools for understanding the biological synthesis and breakdown of the prostaglandins. Synthetic chemists used the structures proposed for the prostaglandins and synthesized large quantities of molecules that had those structures. For his work in designing these challenging syntheses Elias Corey was awarded the Nobel Prize in Chemistry in 1990 (Corey 1990). Later, scientists tested these synthetic prostaglandins in cells and animals and verified that they functioned like the naturally occurring molecules, validating the proposed structures.
Another challenge in solving this puzzle was the enormous range of biological functions researchers found for prostaglandins. These molecules promoted pain and inflammation, regulated pregnancy and child-birth, controlled blood pressure and the secretion of stomach mucus and acid, contracted or relaxed smooth muscle. To make matters even more confusing, some pairs of prostaglandins had actions that opposed each other.
What Else Does the Body Make from the Fatty Acids We Consume?
Prostaglandins were discovered first, but they are not the only signaling molecules synthesized from these essential fatty acids. Thromboxanes, which regulate blood clotting, and leukotrienes, which are important in immune function, are also synthesized using arachidonic acid as a precursor. These diverse signaling molecules derived from essential fatty acids are referred to as eicosanoids — eicos is the Greek root for 20 — since they all originated as 20-carbon fatty acids. Thromboxane, produced in platelets, constricts blood vessels and promotes platelet aggregation, an early step in blood clotting. Leukotrienes attract immune cells (such as neutrophils) to sites of inflammation, constrict bronchioles in the lungs, and make capillary walls permeable. (Samuelsson 1983). Some medical treatments interfere with these actions, as a way to improve health. As a common example, inhalers that are widely used for asthma treatment deliver the drug montelukast, which blocks the interaction between leukotriene molecules and their receptors to inhibit this process.
What Aspirin Taught us About Prostaglandins
What first suggested to scientists that these varied biological molecules might all come from a common precursor? The answer came from aspirin, a drug many people began using in the early 1900s. Although aspirin and a wide range of other non-steroidal anti-inflammatory drugs (NSAIDs) have been used for decades, the biological basis of their actions was not understood. It was not until the mid 1970s that scientists discovered that aspirin influenced prostaglandin production to reduce pain and inflammation.
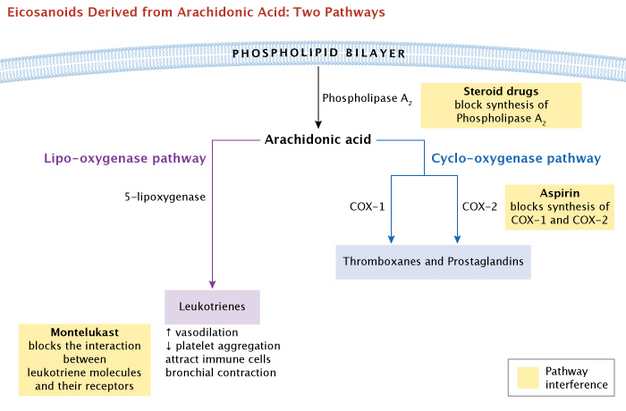
Physicians observed that all these drugs had similar advantages as well as undesired side effects. They could relieve pain and inflammation, but they also caused stomach ulcers and slowed blood clotting. Research by Jon Vane and his lab indicated that aspirin blocked the synthesis of both prostaglandins and thromboxanes (Vane 1971, 1982). This provided a logical explanation for aspirin's dual effect as both 1) a pain reliever and 2) an inhibitor of blood clots. Cyclo-oygenase (COX) was the key enzyme that these drugs inhibited. Later, two forms of COX were identified, and it was possible to selectively inhibit each of them. For a general summary overview, the major branching pathways that lead from arachidonic acid to the eicosanoids are shown in Figure 5. Note that the COX enzymes are positioned under one major branching pathway, the cyclo-oxygenase pathway. Next, we will elaborate on this pathway.
What Systems Do These Signaling Molecules Activate?
The eicosenoids — prostaglandins, thromboxanes, and leukotrienes — are universally produced, and universally active in the body. Virtually every type of cell (other than red blood cells) produces one or more of these signaling molecules. Their very short half-lives means that they are very effective at signaling nearby cells, but fade fast enough to not have global body effects. As these signaling molecules are so potent, their production is tightly regulated.
What major molecules are involved? Normally, the precursor molecule arachidonic acid is stored as a component of phospholipids on the cytoplasmic side of the plasma membrane. Specific phospholipase enzymes, such as phospholipase A, cleave the arachidonic acid away from the phospholipid, which allows the COX enzymes to start the conversion process. This cyclo-oxygenase pathway leads to a potential vairety of effects via the COX enzymes, on many different tissues of the body (Figure 6).
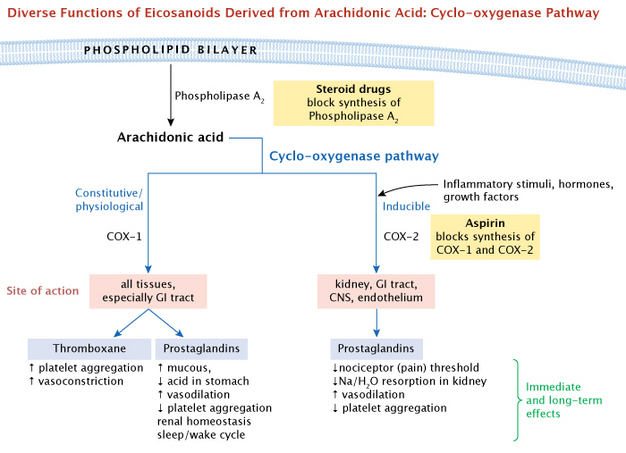
We have already mentioned some drugs that interfere with eicosanoids or their precursors, the COX enzymes. In addition, steroid drugs have a general effect on eicosanoid activity becuase they act at the root of the major branching pathways. These drugs function by blocking synthesis of the enzymes used to release these fatty acids from the cell membrane, preventing the synthesis of prostaglandins. Steroid drugs are commonly used to combat inflammation and are longer acting than other drugs mentioned here.
How do these molecules activate signaling pathways in their target cells? They bind to cell-surface proteins of the seven-helix-G protein-coupled receptors (GPCRs). These in turn activate either the cyclic AMP pathway or the inositol phosphate-calcium pathway. Altogether, these cascading pathways can produce both immediate and long-term changes in their target cells.
How Are These Fatty Acids Involved in the Effects of Marijuana?
What is the Function of These Fatty Acid Derived Molecules in Non-Animal Life Forms?
One such molecule is jasmonic acid — and its volatile derivative, methyl jasmonate — which are important components of the plant's reaction to attack (Figure 8). Jasmonic acids are also made by starting with the carbon skeleton of linolenic acid, in a pathway that is similar (but not identical) to the pathway that produces prostaglandins in humans. (Turner et al. 2002) Plant cells exposed to jasmonic acid increase the production of several antibacterial and antifungal proteins in their cells. In this case, plants have one important advantage over animals as they contain the enzymes necessary to add the specific double bonds needed to produce linolenic, arachidonic acid, and other long-chain polyunsaturated fatty acids. Unlike animals, however, plants synthesize all of their essential molecules from carbon dioxide, minerals from soil, and sunlight. These observations do pose an interesting question — why were the same set of starting molecules, unsaturated fatty acids, chosen as the starting point for signaling molecules in these widely divergent organisms?
Our Day-to-Day Diet
How much of these fats do you need? Current recommendations suggest around 1–1.5g of omega-3 fat (such as linoleic acid) and 10–15g of omega-6 fats (such as linolenic acid) per day for adults (Food and Nutrition Board 2002). Currently, there is a huge interest in the role of specific fats in human health, as they make headline news nearly every day. Some of the most compelling hypotheses for how different types of dietary fat could influence such diverse processes as asthma, cardiovascular disease, and brain development, suggest that these diverse fats, from the long-chain unsaturated fats found in fish oil to the trans fats found in processed food, work by altering these lipid based signaling systems. The amount and ratios of these fats in our diet could increase or decrease the synthesis of prostaglandins, thromboxanes, and leukotrienes in our bodies. They could also increase or decrease the effects of these signals on their normal target cells. The reactions that produce these signaling molecules turn out to be those blocked by the action of drugs such as aspirin. Similar signaling molecules derived from fatty acids are found in diverse organisms. How our diet influences these pathways, whether by intake of trans fats or the ratio of omega-6 to omega-3 fatty acids, is currently the subject of great research interest because of its possible impact on all aspects of human health, from the neural development of children to cardiovascular disease in adults.
References and Recommended Reading
Bergstrom, S. K. The Prostaglandins: From the Laboratory to the Clinic. (1982).
Burr, G. O. & Burr, M. M. A new deficiency disease produced by the rigid exclusion of fat from the diet. Journal of Biological Chemistry 82, 345–367 (1929).
Burr, G.O., Burr, M. M. et al. On the Fatty Acids Essential in Nutrition. III .Journal of Biological Chemistry 97 1–9 (1932).
Corey, E. The logic of chemical synthesis: multistep synthesis of complex carbogenic molecules (1990).
Devane, W. A., Hanus, L., et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992). doi: 10.1126/science.1470919.
FitzGerald, G. A. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nature Reviews Drug Discovery 2, 879-890 (2003) doi:10.1038/nrd1225.
Food and Nutrition Board, Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002).
Justice, E. & Carruthers, D. M. Cardiovascular risk and COX-2 inhibition in rheumatological practice. Journal of Human Hypertension 19, 1-5 (2005). doi:10.1038/sj.jhh.1001777.
Vane, J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (New Biology) 231, 232–235 (1971). doi: 10.1038/10.1038/newbio231232a0.
Vane, J. R. Adventures and Excursions in Bioassay: The Stepping Stones to Prostacyclin (1982).
Samuelsson, B. From Studies of Biochemical Mechanisms to Novel Biological Mediators: Prostaglandin Endoperoxides, Thromboxanes and Leukotrienes (1982).
Samuelsson, B. Leukotrienes: mediators of immediate hypersensitivity, reactions and inflammation, Science 220 568–575 (1983) doi: 10.1126/science.6301011.
Turner, J. G., Ellis, C., et al. The jasmonate signal pathway. The Plant Cell 14, S153–s164 (2002). doi: 10.1105/tpc.000679.



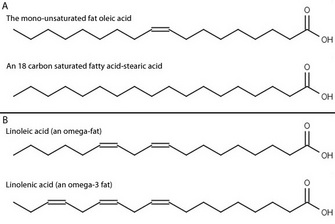
 Figure 2: The structure of the fatty acids examined
Figure 2: The structure of the fatty acids examined