« Prev Next »

Endoplasmic Reticulum, Golgi Apparatus, and Lysosomes
Cells have extensive sets of intracellular membranes, which together compose the endomembrane system. The endomembrane system was first discovered in the late 1800s when scientist Camillo Golgi noticed that a certain stain selectively marked only some internal cellular membranes. Golgi thought that these intracellular membranes were interconnected, but advances in microscopy and biochemical studies of the various membrane-encased organelles later made it clear the organelles in the endomembrane system are separate compartments with specific functions. These structures do exchange membrane material, however, via a special type of transport.
Today, scientists know that the endomembrane system includes the endoplasmic reticulum (ER), Golgi apparatus, and lysosomes. Vesicles also allow the exchange of membrane components with a cell's plasma membrane.
How Are Cell Membranes Synthesized?
Membranes and their constituent proteins are assembled in the ER. This organelle contains the enzymes involved in lipid synthesis, and as lipids are manufactured in the ER, they are inserted into the organelle's own membranes. This happens in part because the lipids are too hydrophobic to dissolve into the cytoplasm.
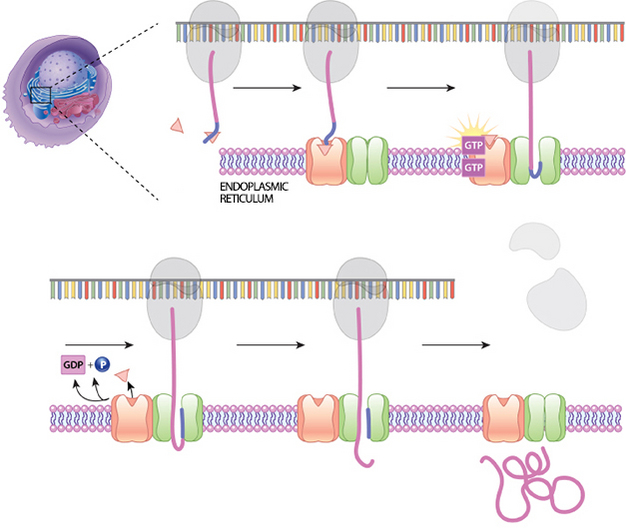
Similarly, transmembrane proteins have enough hydrophobic surfaces that they are also inserted into the ER membrane while they are still being synthesized. Here, future membrane proteins make their way to the ER membrane with the help of a signal sequence in the newly translated protein. The signal sequence stops translation and directs the ribosomes — which are carrying the unfinished proteins — to dock with ER proteins before finishing their work. Translation then recommences after the signal sequence docks with the ER, and it takes place within the ER membrane. Thus, by the time the protein achieves its final form, it is already inserted into a membrane (Figure 1).
The proteins that will be secreted by a cell are also directed to the ER during translation, where they end up in the lumen, the internal cavity, where they are then packaged for vesicular release from the cell. The hormones insulin and erythropoietin (EPO) are both examples of vesicular proteins.
How Are Organelle Membranes Maintained?
The ER, Golgi apparatus, and lysosomes are all members of a network of membranes, but they are not continuous with one another. Therefore, the membrane lipids and proteins that are synthesized in the ER must be transported through the network to their final destination in membrane-bound vesicles. Cargo-bearing vesicles pinch off of one set of membranes and travel along microtubule tracks to the next set of membranes, where they fuse with these structures. Trafficking occurs in both directions; the forward direction takes vesicles from the site of synthesis to the Golgi apparatus and next to a cell's lysosomes or plasma membrane. Vesicles that have released their cargo return via the reverse direction. The proteins that are synthesized in the ER have, as part of their amino acid sequence, a signal that directs them where to go, much like an address directs a letter to its destination.
Soluble proteins are carried in the lumens of vesicles. Any proteins that are destined for a lysosome are delivered to the lysosome interior when the vesicle that carries them fuses with the lysosomal membrane and joins its contents. In contrast, the proteins that will be secreted by a cell, such as insulin and EPO, are held in storage vesicles. When signaled by the cell, these vesicles fuse with the plasma membrane and release their contents into the extracellular space.
What Does the Golgi Apparatus Do?
The Golgi apparatus functions as a molecular assembly line in which membrane proteins undergo extensive post-translational modification. Many Golgi reactions involve the addition of sugar residues to membrane proteins and secreted proteins. The carbohydrates that the Golgi attaches to membrane proteins are often quite complex, and their synthesis requires multiple steps.
In electron micrographs, the Golgi apparatus looks like a set of flattened sacs. Vesicles that bud off from the ER fuse with the closest Golgi membranes, called the cis-Golgi. Molecules then travel through the Golgi apparatus via vesicle transport until they reach the end of the assembly line at the farthest sacs from the ER — called the trans-Golgi. At each workstation along the assembly line, Golgi enzymes catalyze distinct reactions. Later, as vesicles of membrane lipids and proteins bud off from the trans-Golgi, they are directed to their appropriate destinations — either lysosomes, storage vesicles, or the plasma membrane (Figure 2).

What Do Lysosomes Do?

Lysosomes are formed by the fusion of vesicles that have budded off from the trans-Golgi. The sorting system recognizes address sequences in the hydrolytic enzymes and directs them to growing lysosomes. In addition, vesicles that bud off from the plasma membrane via endocytosis are also sent to lysosomes, where their contents — fluid and molecules from the extracellular environment — are processed. The process of endocytosis is an example of reverse vesicle trafficking, and it plays an important role in nutrition and immunity as well as membrane recycling. Lysosomes break down and thus disarm many kinds of foreign and potentially pathogenic materials that get into the cell through such extracellular sampling (Figure 3).
Conclusion
eBooks
This page appears in the following eBook





















