« Prev Next »
The energy needs of the human body must be fulfilled despite the fluctuations in nutrient availability that the body experiences on a daily basis. How, then, do our different cells use fuel molecules, and what factors are involved in this process? We can think of the human body as a dynamic environment where each cell has to continually and sometimes cyclically switch the type of substrate that is oxidized and/or produced. This adaptation is crucial and is achieved only through the several regulatory mechanisms involved in controlling energy transformation and utilization. Moreover, cellular adaptation becomes more crucial when we consider the diverse physiological conditions an organism is exposed to on a daily basis. For example, during the night we usually do not eat, a type of "fasting" that is later disrupted by breakfast, and at other times we are simply resting, or exercising. In these situations, the type and amount of nutrients available for cells change abruptly.
Energy Metabolism and ATP Synthesis in Human Cells
Mitochondria are the main site for ATP synthesis in mammals, although some ATP is also synthesized in the cytoplasm. Lipids are broken down into fatty acids, proteins into amino acids, and carbohydrates into glucose. Via a series of oxidation-reduction reactions, mitochondria degrade fatty acids, amino acids, and pyruvate (the end product of glucose degradation in the cytoplasm) into several intermediate compounds, as well as into the reduced electron carrier coenzymes NADH and FADH2 (Figure 1). The intermediates enter the tricarboxylic acid (TCA) cycle, also giving rise to NADH and FADH2. These reduced electron carriers are themselves oxidized via the electron transport chain, with concomitant consumption of oxygen and ATP synthesis (Figure 1). This process is called oxidative phosphorylation.
Over a hundred ATP molecules are synthesized from the complete oxidation of one molecule of fatty acid, and almost forty ATP molecules result from amino acid and pyruvate oxidation. Two ATP molecules are synthesized in the cytoplasm via the conversion of glucose molecules to pyruvate. Both the apparatus (enzymes) and the physical environment necessary for the oxidation of these molecules are contained in the mitochondria.
Different Cell Types Require Different Fuel Molecules
In fact, many different cells do oxidize fatty acids for ATP production (Figure 2). Between meals, cardiac muscle cells meet 90% of their ATP demands by oxidizing fatty acids. Although these proportions may fall to about 60% depending on the nutritional status and the intensity of contractions, fatty acids may be considered the major fuel consumed by cardiac muscle. Skeletal muscle cells also oxidize lipids. Indeed, fatty acids are the main source of energy in skeletal muscle during rest and mild-intensity exercise. As exercise intensity increases, glucose oxidation surpasses fatty acid oxidation. Other secondary factors that influence the substrate of choice for muscle include exercise duration, gender, and training status.
Another tissue that utilizes fatty acids in high amount is adipose tissue. Since adipose tissue is the storehouse of body fat, one might conclude that, during fasting, the source of fatty acids for adipose tissue cells is their own stock. Skeletal muscle and adipose tissue cells also utilize glucose in significant proportions, but only at the absorptive stage - that is, right after a regular meal. Other organs that use primarily fatty acid oxidation are the kidney and the liver. The cortex cells of the kidneys need a constant supply of energy for continual blood filtration, and so does the liver to accomplish its important biosynthetic functions.
Despite their massive use as fuels, fatty acids are oxidized only in the mitochondria. But not all human cells possess mitochondria! Although that may sound strange, human red blood cells are the most common cells lacking mitochondria. Other examples include tissues of the eyes, such as the lens, which is almost totally devoid of mitochondria; and the outer segment of the retina, which contains the photosensitive pigment. You may have already guessed that these cells and tissues then must produce ATP by metabolizing glucose only. In these situations, glucose is degraded to pyruvate, which is then promptly converted to lactate (Figure 2). This process is called lactic acid fermentation. Although not highly metabolically active, red blood cells are abundant, resulting in the continual uptake of glucose molecules from the bloodstream. Additionally, there are cells that, despite having mitochondria, rely almost exclusively on lactic acid fermentation for ATP production. This is the case for renal medulla cells, whose oxygenated blood supply is not adequate to accomplish oxidative phosphorylation.
Finally, what if the availability of fatty acids to cells changes? The blood-brain barrier provides a good example. In most physiological situations, the blood-brain barrier prevents the access of lipids to the cells of the central nervous system (CNS). Therefore, CNS cells also rely solely on glucose as fuel molecules (Figure 2). In prolonged fasting, however, ketone bodies released in the blood by liver cells as part of the continual metabolization of fatty acids are used as fuels for ATP production by CNS cells. In both situations and unlike red blood cells, however, CNS cells are extremely metabolically active and do have mitochondria. Thus, they are able to fully oxidize glucose, generating greater amounts of ATP. Indeed, the daily consumption of nerve cells is about 120 g of glucose equivalent, which corresponds to an input of about 420 kilocalories (1,760 kilojoules). This figure accounts for 60% of glucose utilization (or 20% of the energy needs of the human body in the resting state). However, most remaining cell types in the human body have mitochondria, adequate oxygen supply, and access to all three fuel molecules. Which fuel, then, is preferentially used by each of these cells?
The Type of Fuel Molecule Changes according to Cell Function and Physiological Context
Virtually all cells are able to take up and utilize glucose. What regulates the rate of glucose uptake is primarily the concentration of glucose in the blood. Glucose enters cells via specific transporters (GLUTs) located in the cell membrane. There are several types of GLUTs, varying in their location (tissue specificity) and in their affinity for glucose. Adipose and skeletal muscle tissues have GLUT4, a type of GLUT which is present in the plasma membrane only when blood glucose concentration is high (e.g., after a carbohydrate-rich meal). The presence of this type of transporter in the membrane increases the rate of glucose uptake by twenty- to thirtyfold in both tissues, increasing the amount of glucose available for oxidation. Therefore, after meals glucose is the primary source of energy for adipose tissue and skeletal muscle.
The breakdown of glucose, in addition to contributing to ATP synthesis, generates compounds that can be used for biosynthetic purposes. So the choice of glucose as the primary oxidized substrate is very important for cells that can grow and divide fast. Examples of these cell types include white blood cells, stem cells, and some epithelial cells.
A similar phenomenon occurs in cancer cells, where increased glucose utilization is required as a source of energy and to support the increased rate of cell proliferation. Interestingly, across a tumor mass, interior cells may experience fluctuations in oxygen tension that in turn limit nutrient oxidation and become an important aspect for tumor survival. In addition, the increased glucose utilization generates high amounts of lactate, which creates an acidic environment and facilitates tumor invasion.
Another factor that dramatically affects the metabolism is the nutritional status of the individual — for instance, during fasting or fed states. After a carbohydrate-rich meal, blood glucose concentration rises sharply and a massive amount of glucose is taken up by hepatocytes by means of GLUT2. This type of transporter has very low affinity for glucose and is effective only when glucose concentration is high. Thus, during the fed state the liver responds directly to blood glucose levels by increasing its rate of glucose uptake. In addition to being the main source of energy, glucose is utilized in other pathways, such as glycogen and lipid synthesis by hepatocytes. The whole picture becomes far more complex when we consider how hormones influence our energy metabolism. Fluctuations in blood levels of glucose trigger secretion of the hormones insulin and glucagon. How do such hormones influence the use of fuel molecules by the various tissues?
Hormones Regulate Cell Metabolism
Human cells and tissues adapt to internal metabolic demands in many ways, mostly in response to hormones and/or nervous stimuli. Demands by one cell type can be met by the consumption of its own reserves and by the uptake of fuel molecules released in the bloodstream by other cells. Energy use is tightly regulated so that the energy demands of all cells are met simultaneously. Elevated levels of glucose stimulate pancreatic β-cells to release insulin into the bloodstream. Virtually all cells respond to insulin; thus, during the fed state cell metabolism is coordinated by insulin signaling.
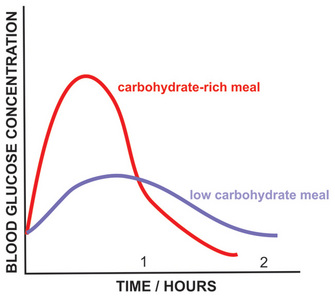
The Liver Supplies Blood Glucose
The liver is a very active organ that performs different vital functions. In Greek mythology, Prometheus steals fire from Zeus and gives it to mortals. As a punishment, Zeus has part of Prometheus's liver fed to an eagle every day. Since the liver grows back, it is eaten repeatedly. This story illustrates the high proliferative rate of liver cells and the vital role of this organ for human life. One of its most important functions is the maintenance of blood glucose. The liver releases glucose by degrading its glycogen stores. This reserve is not large, and during overnight fasting glycogen reserves fall severely. Glycogen stores in the liver correspond to 6% of its mass. On the other hand, glycogen stores in the muscle correspond to 1% of muscle mass but represent three to four times the amount found in liver, since by mass we have more muscle than liver. However, only the liver supplies the blood with glucose since it has an enzyme that make it possible for glucose molecules to be transported across cell membranes.
Since glycogen stores are limited and are reduced within 12-18 hours of fasting, and blood glucose concentration is kept within narrow limits under most physiological conditions, another mechanism must exist to supply blood glucose. Indeed, glucose can be synthesized from amino acid molecules. This process is called de novo synthesis of glucose, or gluconeogenesis. Amino acids, while being degraded, generate several intermediates that are used by the liver to synthesize glucose (Figure 2). Alanine and glutamine are the two amino acids whose main function is to contribute to glucose synthesis by the liver. The kidneys also possess the enzymes necessary for gluconeogenesis and, during prolonged fasting, contribute to some extent to the supply of blood glucose. Furthermore, since de novo glucose synthesis comes from amino acid degradation and the depletion of protein stores can be life-threatening, this process must be regulated. Insulin, glucagon, and another hormone, glucocorticoid, play important roles in controlling the rate of protein degradation and, therefore, the rate of glucose production by the liver.
Summary
Alterations in factors that control food intake and regulate energy metabolism are related to well-known pathological conditions such as obesity, type 2 diabetes and the metabolic syndrome, and some types of cancer. In addition, many effects and regulatory actions of well-known hormones such as insulin are still poorly understood. The consideration of adipose tissue as a dynamic and active tissue, for instance, raises several important issues regarding body weight and the control of food intake. These factors point to the importance of further studies to expand our understanding of energy metabolism, thereby improving our quality of life and achieving a comprehensive view of how the human body functions.
References and Recommended Reading
Cahill, G. F., Jr. Fuel metabolism in starvation. Annual Review of Nutrition 26, 1–22 (2006)
Iyer, A., et al. Inflammatory lipid mediators in adipocyte function and obesity. Nature Reviews Endocrinology 6, 71–82 (2010)
Kaelin, W. G., Jr., & Thompson, C. B. Q&A: Cancer: Clues from cell metabolism Nature 3, 562–564 (2010)
Kodde, I. F., et al. Metabolic and genetic regulation of cardiac energy substrate preference. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 146, 26–39 (2007)
Kresge, N., Simoni, R. D., & Hill, R. L. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. Journal of Biological Chemisry 280, e3 (2005)
Kroemer, G., & Pouyssegur, J. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell 13, 472–482 (2008)
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. Understanding the Warburg effect: The metabolic requirements of cell proliferation Science 22, 1029–1033 (2009)
van der Vusse, G. J., et al. Critical steps in cellular fatty acid uptake and utilization. Molecular and Cellular Biochemistry 239, 9–15 (2002)



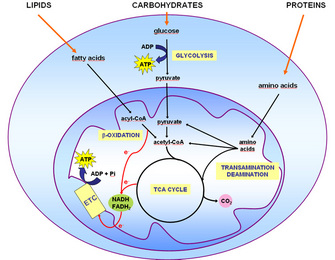
 Figure 1: Schematic representation of fuel molecule entry points in oxidative metabolism
Figure 1: Schematic representation of fuel molecule entry points in oxidative metabolism



























