Key Points
-
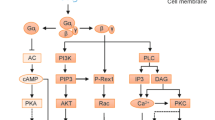
G protein-coupled receptors (GPCRs) are transmembrane receptor proteins involved in many pathophysiological processes in rheumatic diseases
-
GPCRs include a wide variety of receptors relevant to arthritis pathophysiology, including adenosine, chemokines, kinin B2, complement factor, and protease-activated receptors
-
Methotrexate, the most commonly used drug to treat rheumatoid arthritis, works via the adenosine A2A GPCR signalling pathway
-
Therapeutic approaches that target GPCRs could help in reducing the disease progression of a number of rheumatic diseases
Abstract
G protein-coupled receptors (GPCRs) are transmembrane receptor proteins that allow the transfer of signals across the cell membrane. In addition to their physiological role, GPCRs are involved in many pathophysiological processes including pathways relevant in rheumatoid arthritis (RA), osteoarthritis (OA) and psoriatic arthritis. Two-thirds of all currently available drugs target GPCRs directly or indirectly. However, the detailed mechanism of GPCR signalling is still unclear. Selective modification of GPCR-dependent signalling cascades to inhibit disease progression in rheumatic diseases is now being investigated. One approach is to use antibodies against ligands activating GPCRs. However, several GPCRs are known to be activated by only one ligand. In this case, targeting the receptor itself is a promising approach. So far, more information is available on GPCR action in RA as compared with OA, and even less information is available for other rheumatic diseases. Additional research on the role of GPCRs involved in the pathophysiology of rheumatic diseases is required to develop specific therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002).
Venkatakrishnan, A. J. et al. Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013).
Hutchings, C. J., Koglin, M. & Marshall, F. H. Therapeutic antibodies directed at G protein-coupled receptors. MAbs 2, 594–606 (2010).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000).
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Katritch, V., Cherezov, V. & Stevens, R. C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 (2013).
Katritch, V., Cherezov, V. & Stevens, R. C. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 33, 17–27 (2012).
Steyaert, J. & Kobilka, B. K. Nanobody stabilization of G protein-coupled receptor conformational states. Curr. Opin. Struct. Biol. 21, 567–572 (2011).
Latek, D., Modzelewska, A., Trzaskowski, B., Palczewski, K. & Filipek, S. G protein-coupled receptors—recent advances. Acta Biochim. Pol. 59, 515–529 (2012).
Giguere, P. M., Laroche, G., Oestreich, E. A. & Siderovski, D. P. G-protein signaling modulator-3 regulates heterotrimeric G-protein dynamics through dual association with Gβ and Gαi protein subunits. J. Biol. Chem. 287, 4863–4874 (2012).
Wettschureck, N. & Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 (2005).
Zhao, A. et al. Gαo represses insulin secretion by reducing vesicular docking in pancreatic β-cells. Diabetes 59, 2522–2529 (2010).
Choudhury, S. R., Bisht, N. C., Thompson, R., Todorov, O. & Pandey, S. Conventional and novel Gγ protein families constitute the heterotrimeric G-protein signaling network in soybean. PLoS One 6, e23361 (2011).
Shukla, A. K. et al. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141 (2013).
Lombardi, M. S. et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 13, 715–725 (1999).
Lombardi, M. S. et al. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J. Immunol. 166, 1635–1640 (2001).
Frommer, K. W. et al. Adiponectin-mediated changes in effector cells involved in the pathophysiology of rheumatoid arthritis. Arthritis Rheum. 62, 2886–2899 (2010).
Penela, P. et al. G protein-coupled receptor kinase 2 (GRK2) in migration and inflammation. Arch. Physiol. Biochem. 114, 195–200 (2008).
Ohta, A. & Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 (2001).
Kinsel, J. F. & Sitkovsky, M. V. Possible targeting of G protein coupled receptors to manipulate inflammation in vivo using synthetic and natural ligands. Ann. Rheum. Dis. 62 (Suppl. 2), ii22–ii24 (2003).
Suarez-Almazor, M. E., Belseck, E., Shea, B., Wells, G. & Tugwell, P. Methotrexate for rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. Art. No.: CD000957. http://dx.doi.org/10.1002/14651858.CD000957.
Wessels, J. A., Huizinga, T. W. & Guchelaar, H. J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 47, 249–255 (2008).
Tian, H. & Cronstein, B. N. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull. NYU Hosp. Jt Dis. 65, 168–173 (2007).
Montesinos, M. C. et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 48, 240–247 (2003).
Jacobson, K. A., Balasubramanian, R., Deflorian, F. & Gao, Z. G. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal. 8, 419–436 (2012).
David, M. et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J. Eur. Acad. Dermatol. Venereol. 26, 361–367 (2012).
Murphy, P. M. et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176 (2000).
Szekanecz, Z., Vegvari, A., Szabo, Z. & Koch, A. E. Chemokines and chemokine receptors in arthritis. Front. Biosci. (Schol. Ed.) 2, 153–167 (2010).
Neumann, E., Lefevre, S., Zimmermann, B., Gay, S. & Müller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 16, 458–468 (2010).
O'Boyle, G., Mellor, P., Kirby, J. A. & Ali, S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding CXCL12. FASEB J. 23, 3906–3916 (2009).
Johnson, Z. et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J. Immunol. 173, 5776–5785 (2004).
Ali, S. et al. A non-glycosaminoglycan-binding variant of CC chemokine ligand 7 (monocyte chemoattractant protein-3) antagonizes chemokine-mediated inflammation. J. Immunol. 175, 1257–1266 (2005).
Ali, S., Fritchley, S. J., Chaffey, B. T. & Kirby, J. A. Contribution of the putative heparan sulfate-binding motif BBXB of RANTES to transendothelial migration. Glycobiology 12, 535–543 (2002).
Yellin, M. et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 64, 1730–1739 (2012).
Hatse, S., Princen, K., Bridger, G., De Clercq, E. & Schols, D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 527, 255–262 (2002).
Garcia-Vicuna, R. et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 50, 3866–3877 (2004).
Chen, H. T. et al. Stromal cell-derived factor-1/CXCR4 promotes IL-6 production in human synovial fibroblasts. J. Cell. Biochem. 112, 1219–1227 (2011).
Matthys, P. et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-γ receptor-deficient mice. J. Immunol. 167, 4686–4692 (2001).
Li, Y. et al. Influence on matrix metalloproteinases 3, 9, and 13 levels after blocking stromal cell derived factor 1/chemokine receptor 4 signaling pathway with AMD3100 [Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26, 652–656 (2012).
Wei, F. et al. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res. Ther. 14, R177 (2012).
Vergunst, C. E. et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939 (2008).
Fleishaker, D. L. et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res. Ther. 14, R11 (2012).
Singh, M. D. et al. Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. Am. J. Pathol. 181, 1158–1164 (2012).
Lindberg, M. Use of NSAIDs in rheumatoid arthritis should be limited [Danish]. Ugeskr. Laeger 175, 1039–1041 (2013).
Kinsey, S. G., Naidu, P. S., Cravatt, B. F., Dudley, D. T. & Lichtman, A. H. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol. Biochem. Behav. 99, 718–725 (2011).
Laragione, T., Brenner, M., Sherry, B. & Gulko, P. S. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion in rheumatoid arthritis. Arthritis Rheum. 63, 3274–3283 (2011).
Chao, P. Z., Hsieh, M. S., Cheng, C. W., Lin, Y. F. & Chen, C. H. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J. Biomed. Sci. 18, 86 (2011).
Flytlie, H. A. et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine 49, 24–29 (2010).
Bellucci, F. et al. Novel effects mediated by bradykinin and pharmacological characterization of bradykinin B2 receptor antagonism in human synovial fibroblasts. Br. J. Pharmacol. 158, 1996–2004 (2009).
Leeb-Lundberg, L. M., Marceau, F., Muller-Esterl, W., Pettibone, D. J. & Zuraw, B. L. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 57, 27–77 (2005).
Song, I. H. et al. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor 2 antagonist in painful knee osteoarthritis compared with MRI. Ann. Rheum. Dis. 68, 75–83 (2009).
Meini, S. et al. Fasitibant prevents the bradykinin and interleukin 1β synergism on prostaglandin E2 release and cyclooxygenase 2 expression in human fibroblast-like synoviocytes. Naunyn Schmiedebergs Arch. Pharmacol. 385, 777–786 (2012).
Sarma, J. V. & Ward, P. A. New developments in C5a receptor signaling. Cell Health Cytoskelet. 4, 73–82 (2012).
Fairlie, D. & Sheils, I. A. Treatment of osteoarthritis: EP 1575606 A1 [online], (2005).
Rosen, H., Stevens, R. C., Hanson, M., Roberts, E. & Oldstone, M. B. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82, 637–662 (2013).
Tsunemi, S. et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 136, 197–204 (2010).
Zu Heringdorf, D. M., Ihlefeld, K. & Pfeilschifter, J. Pharmacology of the sphingosine-1-phosphate signalling system. Handb. Exp. Pharmacol. 215, 239–253 (2013).
Graler, M. H. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell. Physiol. Biochem. 26, 79–86 (2010).
Ferrell, W. R., Kelso, E. B., Lockhart, J. C., Plevin, R. & McInnes, I. B. Protease-activated receptor 2: a novel pathogenic pathway in a murine model of osteoarthritis. Ann. Rheum. Dis. 69, 2051–2054 (2010).
Chen, T. L. et al. Anti-inflammatory mechanisms of the proteinase-activated receptor 2-inhibiting peptide in human synovial cells. J. Biomed. Sci. 18, 43 (2011).
Author information
Authors and Affiliations
Contributions
E.N., K.K. and U.M.-L. contributed equally to researching data for article, substantial contribution to discussion of content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Neumann, E., Khawaja, K. & Müller-Ladner, U. G protein-coupled receptors in rheumatology. Nat Rev Rheumatol 10, 429–436 (2014). https://doi.org/10.1038/nrrheum.2014.62
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.62
This article is cited by
-
CC chemokines and receptors in osteoarthritis: new insights and potential targets
Arthritis Research & Therapy (2023)
-
Proteomic analysis of knee cartilage reveals potential signaling pathways in pathological mechanism of Kashin-Beck disease compared with osteoarthritis
Scientific Reports (2020)
-
Complexities in Genetics of Psoriatic Arthritis
Current Rheumatology Reports (2020)
-
Analysis of gene expression profiles and protein-protein interaction networks in multiple tissues of systemic sclerosis
BMC Medical Genomics (2019)
-
Proteinases and their receptors in inflammatory arthritis: an overview
Nature Reviews Rheumatology (2018)