Key Points
-
Integrated genomic analyses with high-throughput technologies have pointed towards markers that allow for molecular classification of gliomas and for estimations of prognosis. Such markers may become part of the glioma classification and grading system and will also be used for the stratification of clinical trials.
-
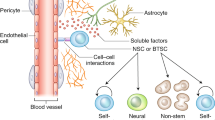
The analysis of tumour initiating cells has pointed towards relationships with normal neuroglial stem cells or progenitors. Attempts to target these cells that are crucial for tumour recurrence must not affect normal cells, and the approach therefore requires that the subtle differences between these cells are defined.
-
Modelling glioma in animals with specific genetic backgrounds of conditional oncogene expressions has pointed towards pathways of glioma development. Convergence of such findings with findings relating to normal stem cell biology and signalling pathways during normal neural development, cell proliferation in the nervous system and neuroregeneration, will lead to a better understanding of the real paths that are involved in neuro-oncological progression.
-
The cellular complexity of high grade glial tumours is increasingly believed to be the result of the recruitment of non-glial cell types into the tumour, adding to the complexity of the oncological target structure.
-
The inaccesability of the infiltrative neoplastic cells beyond the bulk of the tumour and behind the blood–brain barrier calls for complex interstitial therapies, including the use of motile cellular therapies with tumour-specific homing capacities, some of which are derived from the characterization of human neuroglial stem cell properties.
Abstract
Gliomas are the most common type of primary brain tumour and are often fast growing with a poor prognosis for the patient. Their complex cellular composition, diffuse invasiveness and capacity to escape therapies has challenged researchers for decades and hampered progress towards an effective treatment. Recent molecular characterization of tumour cells combined with new insights into cellular diversification that occurs during development, and the modelling of these processes in transgenic animals have enabled a more detailed understanding of the events that underlie gliomagenesis. Combining this enhanced understanding of the relationship between neural stem cell biology and the cell lineage relationships of tumour cells with model systems offers new opportunities to develop specific and effective therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ludwin, S. K. Reaction of oligodendrocytes and astrocytes to trauma and implantation. A combined autoradiographic and immunohistochemical study. Lab. Invest. 52, 20–30 (1985).
Neuwelt, E. A. et al. Reversible osmotic blood-brain barrier disruption in humans: implications for the chemotherapy of malignant brain tumors. Neurosurgery 7, 44–52 (1980).
Louis, D. N. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007).
Central Brain Tumor Registry of the United States. Statistical report: primary brain tumors in the United States, 1997–2001 [online] (2004).
Riemenschneider, M. J. & Reifenberger, G. Molecular neuropathology of gliomas. Int. J. Mol. Sci. 10, 184–212 (2009).
Louis, D. N. Molecular pathology of malignant gliomas. Annu. Rev. Pathol. 1, 97–117 (2006).
Miller, C. R. & Perry, A. Glioblastoma. Arch. Pathol. Lab. Med. 131, 397–406 (2007).
Reifenberger, G. & Wesseling, P. Molecular diagnostics of brain tumors. Acta Neuropathol. 120, 549–551 (2010).
Gotz, M. Glial cells generate neurons--master control within CNS regions: developmental perspectives on neural stem cells. Neuroscientist 9, 379–397 (2003).
McDonald, J. M. et al. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer 104, 1468–1477 (2005).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009). A groundbreaking study in which new molecular markers are linked to the classification of glioma.
Hartmann, C. et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 120, 707–718 (2010).
von Deimling, A., Korshunov, A. & Hartmann, C. The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol. 21, 74–87 (2011).
Reitman, Z. J. & Yan, H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J. Natl Cancer Inst. 102, 932–941 (2010).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011). A landmark study in which alteration of the activity of an enzyme that is involved in cellular metabolism is linked to epigenetic gene regulation, with consequences for tumour cell phenotypes.
Christensen, B. C. et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J. Natl Cancer Inst. 103, 143–153 (2011).
Capper, D. et al. Application of mutant IDH1 antibody to differentiate diffuse glioma from nonneoplastic central nervous system lesions and therapy-induced changes. Am. J. Surg. Pathol. 34, 1199–1204 (2010).
Phillips, H. S. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006). A landmark study that incorporates gene expression analysis to further subdivide gliomas. The paper contributes to the histopathological and genetic approaches that are already used for glioma classification.
Verhaak, R. G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Lei, L. et al. Glioblastoma Models Reveal the Connection between Adult Glial Progenitors and the Proneural Phenotype. PLoS ONE 6, e20041 (2011).
Colman, H. et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 12, 49–57 (2010).
Li, A., Bozdag, S., Kotliarov, Y. & Fine, H. A. GliomaPredict: a clinically useful tool for assigning glioma patients to specific molecular subtypes. BMC Med. Inform. Decis. Mak. 10, 38 (2010).
Ovaska, K. et al. Large-scale data integration framework provides a comprehensive view on glioblastoma multiforme. Genome Med. 2, 65 (2010).
Chen, R. et.al. Clonal analysis reveals a hierarchy of self renewing tumor initiating cell types in glioblastoma. Neuro Oncol. Abstr. 10, SC-35 (2008).
Labussiere, M. et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology 74, 1886–1890 (2010).
Giese, A. et al. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J. Neurooncol. 66, 351–360 (2004).
Gilbertson, R. J. & Gutmann, D. H. Tumorigenesis in the brain: location, location, location. Cancer Res. 67, 5579–5582 (2007).
Cayre, M., Canoll, P. & Goldman, J. E. Cell migration in the normal and pathological postnatal mammalian brain. Prog. Neurobiol. 88, 41–63 (2009). An excellent overview of the cell biology of motile cells in the CNS.
Kakita, A. & Goldman, J. E. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron 23, 461–472 (1999).
Hatten, M. E. Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511–539 (1999).
Fricker, R. A. et al. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 19, 5990–6005 (1999).
Suzuki, S. O. & Goldman, J. E. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J. Neurosci. 23, 4240–4250 (2003).
Jacobsen, C. T. & Miller, R. H. Control of astrocyte migration in the developing cerebral cortex. Dev. Neurosci. 25, 207–216 (2003).
Gould, E. How widespread is adult neurogenesis in mammals? Nature Rev. Neurosci. 8, 481–488 (2007).
Nunes, M. C. et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature Med. 9, 439–447 (2003).
Canoll, P. & Goldman, J. E. The interface between glial progenitors and gliomas. Acta Neuropathol. 116, 465–477 (2008).
Siebzehnrubl, F. A., Reynolds, B. A., Vescovi, A., Steindler, D. A. & Deleyrolle, L. P. The origins of glioma: E Pluribus Unum? Glia 59, 1135–1147 (2011). A comprehensive overview of the current thinking about the relationship between neural stem cells and progenitor cells, and their relationship to possible cells of origin for gliomas.
Venere, M., Fine, H. A., Dirks, P. B. & Rich, J. N. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer's hierarchy. Glia 59, 1148–1154 (2011).
Imayoshi, I., Sakamoto, M., Yamaguchi, M., Mori, K. & Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30, 3489–3498 (2010).
Androutsellis-Theotokis, A. et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 (2006).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nature Neurosci. 8, 709–715 (2005).
Iso, T., Kedes, L. & Hamamori, Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237–255 (2003).
Corbin, J. G. et al. Regulation of neural progenitor cell development in the nervous system. J. Neurochem. 106, 2272–2287 (2008).
Wang, L. et al. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 158, 1356–1363 (2009).
Carlen, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nature Neurosci. 12, 259–267 (2009).
Andreu-Agullo, C., Morante-Redolat, J. M., Delgado, A. C. & Farinas, I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nature Neurosci. 12, 1514–1523 (2009).
Muroyama, Y. & Saito, T. Identification of Nepro, a gene required for the maintenance of neocortex neural progenitor cells downstream of Notch. Development 136, 3889–3893 (2009).
Stockhausen, M. T., Kristoffersen, K. & Poulsen, H. S. The functional role of Notch signaling in human gliomas. Neuro Oncol. 12, 199–211 (2010).
Puget, S. et al. Candidate genes on chromosome 9q33-34 involved in the progression of childhood ependymomas. J. Clin. Oncol. 27, 1884–1892 (2009).
Pierfelice, T. J., Schreck, K. C., Eberhart, C. G. & Gaiano, N. Notch, neural stem cells, and brain tumors. Cold Spring Harb. Symp. Quant. Biol. 73, 367–375 (2008).
Xu, P. et al. The oncogenic roles of Notch1 in astrocytic gliomas in vitro and in vivo. J. Neurooncol 97, 41–51 (2009).
Shiras, A. et al. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells 25, 1478–1489 (2007).
Cooper, L. A. et al. The proneural molecular signature is enriched in oligodendrogliomas and predicts improved survival among diffuse gliomas. PLoS ONE 5, e12548 (2010).
Fan, X. et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28, 5–16 (2010).
Lin, J., Zhang, X. M., Yang, J. C., Ye, Y. B. & Luo, S. Q. γ-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 41, 519–529 (2010).
Fan, X. et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 66, 7445–7452 (2006).
Miele, L., Miao, H. & Nickoloff, B. J. NOTCH signaling as a novel cancer therapeutic target. Curr. Cancer Drug Targets. 6, 313–323 (2006).
Gordon, W. R., Arnett, K. L. & Blacklow, S. C. The molecular logic of Notch signaling-a structural and biochemical perspective. J. Cell Sci. 121, 3109–3119 (2008).
Kovall, R. A. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene 27, 5099–5109 (2008).
Gilbert, C. A., Daou, M. C., Moser, R. P. & Ross, A. H. γ-secretase inhibitors enhance temozolomide treatment of human gliomas by inhibiting neurosphere repopulation and xenograft recurrence. Cancer Res. 70, 6870–6879 (2010).
Pannuti, A. et al. Targeting Notch to target cancer stem cells. Clin. Cancer Res. 16, 3141–3152 (2010).
Singh, S. K., Clarke, I. D., Hide, T. & Dirks, P. B. Cancer stem cells in nervous system tumors. Oncogene 23, 7267–7273 (2004). One of the first reports to introduce the stem cell concept in relation to brain tumours.
Boivin, D. et al. The stem cell marker CD133 (prominin-1) is phosphorylated on cytoplasmic tyrosine-828 and tyrosine-852 by Src and Fyn tyrosine kinases. Biochemistry 48, 3998–4007 (2009).
Lendahl, U., Zimmerman, L. B. & McKay, R. D. CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–595 (1990).
Komitova, M. & Eriksson, P. S. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci. Lett. 369, 24–27 (2004).
Sakakibara, S. et al. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230–242 (1996).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Griguer, C. E. et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE 3, e3655 (2008).
Soeda, A. et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene 28, 3949–3959 (2009).
Clement, V., Dutoit, V., Marino, D., Dietrich, P. Y. & Radovanovic, I. Limits of CD133 as a marker of glioma self-renewing cells. Int. J. Cancer 125, 244–248 (2009).
Laks, D. R. et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells 27, 980–987 (2009).
Nishide, K., Nakatani, Y., Kiyonari, H. & Kondo, T. Glioblastoma formation from cell population depleted of Prominin1-expressing cells. PLoS ONE 4, e6869 (2009).
Chen, R. et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 17, 362–375 (2010).
Nagarajan, R. P. & Costello, J. F. Epigenetic mechanisms in glioblastoma multiforme. Semin. Cancer Biol. 19, 188–197 (2009).
Esteller, M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21, 5427–5440 (2002).
Fouse, S. D. et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2, 160–169 (2008).
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17, 510–522. An important study emphasizing the relevance of epigenetic modification patterns to glioma phenotypes.
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma N. Engl. J. Med. 352, 997–1003 (2005).
Martinez, R. et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics 4, 255–264 (2009).
Morey, L. & Helin, K. Polycomb group protein-mediated repression of transcription. Trends Biochem. Sci. 35, 323–332 2010.
Kamnasaran, D. & Guha, A. Expression of GATA6 in the human and mouse central nervous system. Brain Res. Dev. Brain Res. 160, 90–95 (2005).
Kamnasaran, D., Qian, B., Hawkins, C., Stanford, W. L. & Guha, A. GATA6 is an astrocytoma tumor suppressor gene identified by gene trapping of mouse glioma model. Proc. Natl Acad. Sci. USA 104, 8053–8058 (2007).
Carro, M. S. et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 463, 318–325 (2009). This study exemplifies the importance of bioinformatics for the analysis of gene expression data to not only identify relevant single genes but also their functional context.
Kashyap, V. et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 18, 1093–1108 (2009).
Cerami, E., Demir, E., Schultz, N., Taylor, B. S. & Sander, C. Automated network analysis identifies core pathways in glioblastoma. PLoS ONE 5, e8918. (2010).
Demuth, T. et al. Glioma cells on the run - the migratory transcriptome of 10 human glioma cell lines. BMC Genomics 9, 54 (2008).
Rao, J. S. Molecular mechanisms of glioma invasiveness: the role of proteases. Nature Rev. Cancer 3, 489–501 (2003).
Levin, V. A. et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J. Neurooncol. 78, 295–302 (2006).
Tervonen, O., Forbes, G., Scheithauer, B. W. & Dietz, M. J. Diffuse “fibrillary” astrocytomas: correlation of MRI features with histopathologic parameters and tumor grade. Neuroradiology 34, 173–178 (1992).
Burger, P. C., Heinz, E. R., Shibata, T. & Kleihues, P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J. Neurosurg. 68, 698–704 (1988).
Brennan, C. et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS ONE 4, e7752 (2009).
Jacques, T. S. et al. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 29, 222–235 (2010).
Dancey, J. & Sausville, E. A. Issues and progress with protein kinase inhibitors for cancer treatment. Nature Rev. Drug Discov. 2, 296–313 (2003).
Rao, S. K., Edwards, J., Joshi, A. D., Siu, I. M. & Riggins, G. J. A survey of glioblastoma genomic amplifications and deletions. J. Neurooncol. 96, 169–179 (2010).
Jackson, E. L. et al. PDGFR α-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51, 187–199 (2006).
Hambardzumyan, D., Amankulor, N. M., Helmy, K. Y., Becher, O. J. & Holland, E. C. Modeling Adult Gliomas Using RCAS/t-va Technology. Transl. Oncol. 2, 89–95 (2009). A paradigmatic paper that illustrates the power of modelling glioma in transgenic animal models.
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009).
Huse, J. T. & Holland, E. C. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nature Rev. Cancer 10, 319–331 (2010).
Dahlstrand, J., Lardelli, M. & Lendahl, U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res. Dev. Brain Res. 84, 109–129 (1995).
Lassman, A. B., Dai, C., Fuller, G. N., Vickers, A. J. & Holland, E. C. Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Akt signaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol. 1, 157–163 (2004).
Lindberg, N., Kastemar, M., Olofsson, T., Smits, A. & Uhrbom, L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene 28, 2266–2275 (2009).
Weiss, W. A. et al. Neuropathology of genetically engineered mice: consensus report and recommendations from an international forum. Oncogene 21, 7453–7463 (2002).
Abel, T. W. et al. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol. Cancer Res. 7, 645–653 (2009).
Wang, Y. et al. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell 15, 514–526 (2009).
de Vries, N. A. et al. Rapid and robust transgenic high-grade glioma mouse models for therapy intervention studies. Clin. Cancer Res. 16, 3431–3441 (2010).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Ligon, K. L., Fancy, S. P., Franklin, R. J. & Rowitch, D. H. Olig gene function in CNS development and disease. Glia 54, 1–10 (2006).
Read, R. D., Cavenee, W. K., Furnari, F. B. & Thomas, J. B. A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 5, e1000374 (2009).
Witte, H. T., Jeibmann, A., Klambt, C. & Paulus, W. Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia 11, 882–888 (2009).
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997).
Bell, A. J., McBride, S. M. & Dockendorff, T. C. Flies as the ointment: Drosophila modeling to enhance drug discovery. Fly 3, 39–49 (2009).
Neumuller, R. A. et al. Genome-wide analysis of self-renewal in Drosophila Neural stem cells by transgenic RNAi. Cell Stem Cell 8, 580–593 (2010).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Faijerson, J. et al. Reactive astrogliosis induces astrocytic differentiation of adult neural stem/progenitor cells in vitro. J. Neurosci. Res. 84, 1415–1424 (2006).
Eddleston, M. & Mucke, L. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neuroscience 54, 15–36 (1993).
Buffo, A. et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl Acad. Sci. USA 105, 3581–3586 (2008).
Mapara, K. Y., Stevenson, C. B., Thompson, R. C. & Ehtesham, M. Stem cells as vehicles for the treatment of brain cancer. Neurosurg. Clin. N. Am. 18, 71–80, ix (2007).
Glass, R. et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 25, 2637–2646 (2005).
Chirasani, S. R. et al. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain 133, 1961–1972 (2010).
Walzlein, J. H. et al. The antitumorigenic response of neural precursors depends on subventricular proliferation and age. Stem Cells 26, 2945–2954 (2008).
Aboody, K. S., Najbauer, J. & Danks, M. K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 15, 739–752 (2008).
Uhl, M. et al. Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem. Biophys. Res. Commun. 328, 125–129 (2005).
Aboody, K., Capela, A., Niazi, N., Stern, J. H. & Temple, S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a rosetta stone. Neuron 70, 597–613 (2011). The most comprehensive recent overview of neural stem cell-based therapies for neurological diseases including glioma.
Sasportas, L. S. et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl Acad. Sci. USA 106, 4822–4827 (2009).
Kinoshita, Y. et al. A gene delivery system with a human artificial chromosome vector based on migration of mesenchymal stem cells towards human glioblastoma HTB14 cells. Neurol. Res. 32, 429–437 (2010).
Menon, L. G. et al. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 27, 2320–2330 (2009).
Sonabend, A. M. et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells 26, 831–841 (2008).
Xu, G. et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol. Int. 33, 466–474 (2009).
Vega, E. A., Graner, M. W. & Sampson, J. H. Combating immunosuppression in glioma. Future Oncol. 4, 433–442 (2008).
Liau, L. M. et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 11, 5515–5525 (2005).
Hau, P. et al. Inhibition of TGF-β2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 17, 201–212 (2007).
Kosztowski, T., Zaidi, H. A. & Quinones-Hinojosa, A. Applications of neural and mesenchymal stem cells in the treatment of gliomas. Expert Rev. Anticancer Ther. 9, 597–612 (2009).
Birnbaum, T. et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J. Neurooncol. 83, 241–247 (2007).
Weimann, J. M., Charlton, C. A., Brazelton, T. R., Hackman, R. C. & Blau, H. M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc. Natl Acad. Sci. USA 100, 2088–2093 (2003).
Johansson, C. B. et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nature Cell Biol. 10, 575–583 (2008).
Graeber, M. B., Scheithauer, B. W. & Kreutzberg, G. W. Microglia in brain tumors. Glia 40, 252–259 (2002).
Brooks, W. H., Markesbery, W. R., Gupta, G. D. & Roszman, T. L. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann. Neurol. 4, 219–224 (1978).
Charles, N. A., Holland, E. C., Gilbertson, R., Glass, R. & Kettenmann, H. The brain tumor microenvironment. Glia 59, 1169–1180 (2010).
Dietrich, J., Han, R., Yang, Y., Mayer-Proschel, M. & Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 5, 22 (2006).
Dietrich, J., Monje, M., Wefel, J. & Meyers, C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 13, 1285–1295 (2008).
Muller, F. J. et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature 455, 401–405 (2008).
Huang, T. T., Sarkaria, S. M., Cloughesy, T. F. & Mischel, P. S. Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics 6, 500–512 (2009).
Lefranc, F. Editorial: on the road to multi-modal and pluri-disciplinary treatment of glioblastomas. Acta Neurochir. 151, 109–112 (2009).
Quant, E. C. & Wen, P. Y. Novel medical therapeutics in glioblastomas, including targeted molecular therapies, current and future clinical trials. Neuroimaging Clin. N. Am. 20, 425–448 (2010).
Yamashita, Y. et al. Convection-enhanced delivery of a topoisomerase I inhibitor (nanoliposomal topotecan) and a topoisomerase II inhibitor (pegylated liposomal doxorubicin) in intracranial brain tumor xenografts. Neuro Oncol. 9, 20–28 (2007).
Lonser, R. R. et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J. Neurosurg. 107, 190–197 (2007).
Weber, F. et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 64, 125–137 (2003).
Kunwar, S. et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 25, 837–844 (2007).
Weaver, M. & Laske, D. W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 65, 3–13 (2003).
Sampson, J. H. et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-α and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 65, 27–35 (2003).
Bidros, D. S., Liu, J. K. & Vogelbaum, M. A. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 6, 117–125 (2010).
Vredenburgh, J. J. et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 25, 4722–4729 (2007).
Iwamoto, F. M. et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73, 1200–1206 (2009).
Martens, T. et al. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin. Cancer Res. 14, 5447–5458 (2008).
Hong, C. et al. Epigenome scans and cancer genome sequencing converge on WNK2, a kinase-independent suppressor of cell growth. Proc. Natl Acad. Sci. USA 104, 10974–10979 (2007).
Rutka, J. T. et al. The evolution and application of techniques in molecular biology to human brain tumors: a 25 year perspective. J. Neurooncol. 92, 261–273 (2009).
Wurdinger, T. et al. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 14, 382–393 (2008).
Martin-Villalba, A., Okuducu, A. F. & von Deimling, A. The evolution of our understanding on glioma. Brain Pathol. 18, 455–463 (2008).
Acknowledgements
The work of M.W. and K.L. in the Hans-Dietrich Herrmann Laboratory for Brain Tumor Biology in the department of Neurosurgery Eppendorf, Germany, has enjoyed the continuous support of the Deutsche Forschungsgemeinschaft (Grants We 928/2-1, 3-1 and 4-1; LA 1300/3-1 and 4-1), the Deutsche Krebshilfe, the Heinrich Bauer Stiftung, the Rickertsen Stiftung, the Monika Kutzner Stiftung, the Roggenbuck-Stiftung and the Bartling Stiftung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Glioma
-
Intrinsic tumour of the brain, originating from any kind of glial cell.
- Anaplasia
-
This describes structural and/or functional alterations in cancer cells in which they revert to an less differentiated state.
- Glioma grading
-
A grading of oncological aggressiveness (anaplasia), from well differentiated to anaplastic.
- Glioblastoma
-
According to the World Health Organization system of classification and grading, this is the most anaplastic tumour of astrocytic lineage — astrocytoma grade IV.
- Glioma stem cell
-
Conceptually, a type of cell that represents the cell from which the tumour was generated, that has the capacity for self-renewal and after gross total surgical removal is responsible for repopulating a recurrent tumour.
- Stem-like cells
-
As stem cells should only be referred to as such if they are omnipotent and have a defined role and specification, cells that have similar properties but that are obtained from a tumour are sometimes called stem-like.
- Stemness
-
This describes the degree to which a cell posesses properties such as self renewal, clonogenicity and capacity for multilineage differentiation.
- Orthotopic
-
This describes xenotransplanted tumours or tumour cells that are placed into the tissue environment — the origin of the implanted tumours. This is in contrast to tumour models that use subcutaneous tumour implantation.
Rights and permissions
About this article
Cite this article
Westphal, M., Lamszus, K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci 12, 495–508 (2011). https://doi.org/10.1038/nrn3060
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3060
This article is cited by
-
m6A-Mediated Upregulation of lncRNA CHASERR Promotes the Progression of Glioma by Modulating the miR-6893-3p/TRIM14 Axis
Molecular Neurobiology (2024)
-
Eukaryotic Extension Factor 2 Kinase may Affect the Occurrence and Development of Glioblastoma Through Immune Cell Infiltration
Neurochemical Research (2022)
-
Sevoflurane inhibits the malignant phenotypes of glioma through regulating miR-146b-5p/NFIB axis
Metabolic Brain Disease (2022)
-
Inhibitory effects of miRNAs in astrocytes on C6 glioma progression via connexin 43
Molecular and Cellular Biochemistry (2021)
-
LncRNA BCYRN1 inhibits glioma tumorigenesis by competitively binding with miR-619-5p to regulate CUEDC2 expression and the PTEN/AKT/p21 pathway
Oncogene (2020)