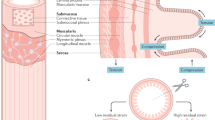
Abstract
Gastrointestinal motility results from coordinated contractions of the tunica muscularis, the muscular layers of the alimentary canal. Throughout most of the gastrointestinal tract, smooth muscles are organized into two layers of circularly or longitudinally oriented muscle bundles. Smooth muscle cells form electrical and mechanical junctions between cells that facilitate coordination of contractions. Excitation–contraction coupling occurs by Ca2+ entry via ion channels in the plasma membrane, leading to a rise in intracellular Ca2+. Ca2+ binding to calmodulin activates myosin light chain kinase; subsequent phosphorylation of myosin initiates cross-bridge cycling. Myosin phosphatase dephosphorylates myosin to relax muscles, and a process known as Ca2+ sensitization regulates the activity of the phosphatase. Gastrointestinal smooth muscles are 'autonomous' and generate spontaneous electrical activity (slow waves) that does not depend upon input from nerves. Intrinsic pacemaker activity comes from interstitial cells of Cajal, which are electrically coupled to smooth muscle cells. Patterns of contractile activity in gastrointestinal muscles are determined by inputs from enteric motor neurons that innervate smooth muscle cells and interstitial cells. Here we provide an overview of the cells and mechanisms that generate smooth muscle contractile behaviour and gastrointestinal motility.
Key Points
-
Gastrointestinal motility occurs by the coordinated contractions of the tunica muscula ris, which forms the outer wall of the alimentary canal from the distal oesophagus to the external anal sphincter
-
Excitation–contraction coupling results from Ca2+ entry into smooth muscle cells, Ca2+ release from the sarcoplasmic reticulum, activation of myosin light chain kinase and phosphorylation of the regulatory light chains of myosin
-
Contractile force is tuned by Ca2+ sensitization mechanisms that balance rates of myosin phosphorylation and dephosphorylation
-
Interstitial cells of Cajal (ICC) provide spontaneous pacemaker activity in gastrointestinal muscles; ICC and PDGFRα+ cells also contribute to mediation of inputs from enteric motor neurons
-
Gastrointestinal motility patterns are highly integrated behaviours requiring coordination between smooth muscle cells and utilizing regulatory inputs from interstitial cells, neurons, and endocrine and immune cells
-
Therapeutic regulation and tissue engineering of gastrointestinal motility is proving difficult
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kiehart, D. P. Molecular genetic dissection of myosin heavy chain function. Cell 60, 347–350 (1990).
Kamm, K. E. & Stull, J. T. Regulation of smooth muscle contractile elements by second messengers. Annu. Rev. Physiol. 51, 299–313 (1989).
Nagai, R., Kuro-o, M., Babij, P. & Periasamy, M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J. Biol. Chem. 264, 9734–9737 (1989).
Kelley, C. A., Takahashi, M., Yu, J. H. & Adelstein, R. S. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J. Biol. Chem. 268, 12848–12854 (1993).
White, S., Martin, A. F. & Periasamy, M. Identification of a novel smooth muscle myosin heavy chain cDNA: isoform diversity in the S1 head region. Am. J. Physiol. 264, C1252–C1258 (1993).
Parisi, J. A. & Eddinger, T. J. Smooth muscle myosin heavy chain isoform distribution in the swine stomach. J. Histochem. Cytochem. 50, 385–393 (2002).
Eddinger, T. J. & Meer, D. P. Myosin II isoforms in smooth muscle: heterogeneity and function. Am. J. Physiol. Cell. Physiol. 293, C493–C508 (2007).
Somlyo, A. P. & Somlyo, A. V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003).
Taylor, D. A. & Stull, J. T. Calcium dependence of myosin light chain phosphorylation in smooth muscle cells. J. Biol. Chem. 263, 14456–14462 (1988).
Niiro, N. & Ikebe, M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 276, 29567–29574 (2001).
Ihara, E. & MacDonald, J. A. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can. J. Physiol. Pharmacol. 85, 79–87 (2007).
Ito, M., Nakano, T., Erdodi, F. & Hartshorne, D. J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 259, 197–209 (2004).
Grassie, M. E., Moffat, L. D., Walsh, M. P. & MacDonald, J. A. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 510, 147–159 (2011).
Mitra, R. & Morad, M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science 229, 269–272 (1985).
Droogmans, G. & Callewaert, G. Ca2+-channel current and its modification by the dihydropyridine agonist BAY K 8644 in isolated smooth muscle cells. Pflügers Arch. 406, 259–265 (1986).
Vogalis, F., Publicover, N. G., Hume, J. R. & Sanders, K. M. Relationship between calcium current and cytosolic calcium concentration in canine gastric smooth muscle cells. Am. J. Physiol. 260, C1012–C1018 (1991).
Bolton, T. B., Prestwich, S. A., Zholos, A. V. & Gordienko, D. V. Excitation–contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 61, 85–115 (1999).
Gibbons, S. J. et al. The alpha1H Ca2+ channel subunit is expressed in mouse jejunal interstitial cells of Cajal and myocytes. J. Cell. Mol. Med. 13, 4422–4431 (2009).
Wang, Y., Deng, X., Hewavitharana, T., Soboloff, J. & Gill, D. L. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol. 35, 1127–1133 (2008).
Tsvilovskyy, V. V. et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137, 1415–1424 (2009).
Venkatachalam, K. & Montell, C. TRP channels. Annu. Rev. Biochem. 76, 387–417 (2007).
Lee, H. K., Shuttleworth, C. W. & Sanders, K. M. Tachykinins activate nonselective cation currents in canine colonic myocytes. Am. J. Physiol. 269, C1394–C1401 (1995).
Inoue, R. & Isenberg, G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 424, 73–92 (1990).
Portbury, A. L., Furness, J. B., Young, H. M., Southwell, B. R. & Vigna, S. R. Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. J. Comp. Neurol. 367, 342–351 (1996).
Grady, E. F. et al. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 16, 6975–6986 (1996).
Iino, S., Ward, S. M. & Sanders, K. M. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J. Physiol. 556, 521–530 (2004).
Sanders, K. M. Regulation of smooth muscle excitation and contraction. Neurogastroenterol. Motil. 20 (Suppl. 1), 39–53 (2008).
Wray, S. & Burdyga, T. Sarcoplasmic reticulum function in smooth muscle. Physiol. Rev. 90, 113–178 (2010).
Sanders, K. M. Invited review: mechanisms of calcium handling in smooth muscles. J. Appl. Physiol. 91, 1438–1449 (2001).
Kotlikoff, M. L., Wang, Y. X., Xin, H. B. & Ji, G. Calcium release by ryanodine receptors in smooth muscle. Novartis Found. Symp. 246, 108–119 (2002).
Kotlikoff, M. I. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog. Biophys. Mol. Biol. 83, 171–191 (2003).
Zhang, L. B., Horowitz, B. & Buxton, I. L. Muscarinic receptors in canine colonic circular smooth muscle. I. Coexistence of M2 and M3 subtypes. Mol. Pharmacol. 40, 943–951 (1991).
Eglen, R. M. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 68, 2573–2578 (2001).
Zhuge, R., Fogarty, K. E., Tuft, R. A. & Walsh, J. V. Jr. Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 microM during a Ca2+ spark. J. Gen. Physiol. 120, 15–27 (2002).
McCarron, J. G., Chalmers, S., Bradley, K. N., MacMillan, D. & Muir, T. C. Ca2+ microdomains in smooth muscle. Cell Calcium 40, 461–493 (2006).
Wellman, G. C. & Nelson, M. T. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34, 211–229 (2003).
Jaggar, J. H., Porter, V. A., Lederer, W. J. & Nelson, M. T. Calcium sparks in smooth muscle. Am. J. Physiol. Cell. Physiol. 278, C235–C256 (2000).
Bolton, T. B. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J. Physiol. 570, 5–11 (2006).
Pacaud, P. & Bolton, T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 441, 477–499 (1991).
Nelson, M. T. et al. Relaxation of arterial smooth muscle by calcium sparks. Science 270, 633–637 (1995).
ZhuGe, R., Sims, S. M., Tuft, R. A., Fogarty, K. E. & Walsh, J. V. Jr. Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J. Physiol. 513, 711–718 (1998).
Bayguinov, O., Hagen, B., Bonev, A. D., Nelson, M. T. & Sanders, K. M. Intracellular calcium events activated by ATP in murine colonic myocytes. Am. J. Physiol. Cell Physiol. 279, C126–C135 (2000).
Dwyer, L. et al. Basally activated nonselective cation currents regulate the resting membrane potential in human and monkey colonic smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G287–G296 (2011).
Large, W. A. & Wang, Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am. J. Physiol. 271, C435–C454 (1996).
McGeown, J. G. Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two? Cell Calcium 35, 613–619 (2004).
Himpens, B. & Casteels, R. Different effects of depolarization and muscarinic stimulation on the Ca2+/force relationship during the contraction-relaxation cycle in the guinea pig ileum. Pflügers Arch. 416, 28–35 (1990).
Kitazawa, T., Eto, M., Woodsome, T. P. & Khalequzzaman, M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J. Physiol. 546, 879–889 (2003).
Hirano, K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J. Pharmacol. Sci. 104, 109–115 (2007).
Haystead, T. A. ZIP kinase, a key regulator of myosin protein phosphatase 1. Cell. Signal. 17, 1313–1322 (2005).
MacDonald, J. A. et al. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc. Natl Acad. Sci. USA 98, 2419–2424 (2001).
Nishimura, J. & van Breemen, C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem. Biophys. Res. Commun. 163, 929–935 (1989).
Wu, X., Somlyo, A. V. & Somlyo, A. P. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem. Biophys. Res. Commun. 220, 658–663 (1996).
Sauzeau, V. et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 275, 21722–21729 (2000).
Shirinsky, V. P. et al. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J. Biol. Chem. 268, 16578–16583 (1993).
Khromov, A. S. et al. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc. Natl Acad. Sci. USA 103, 2440–2445 (2006).
Bennett, M. R., Burnstock, G. & Holman, M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J. Physiol. 182, 541–558 (1966).
Bennett, M. R. Transmission from intramural excitatory nerves to the smooth muscle cells of the guinea-pig taenia coli. J. Physiol. 185, 132–147 (1966).
Bayliss, W. M. & Starling, E. H. The movements and innervation of the small intestine. J. Physiol. 24, 99–143 (1899).
Kosterlitz, H. W., Pirie, V. W. & Robinson, J. A. The mechanism of the peristaltic reflex in the isolated guinea-pig ileum. J. Physiol. 133, 681–694 (1956).
Hirst, G. D. S., Holman, M. E. & McKirdy, H. C. Two descending nerve pathways activated by distension of guinea-pig small intestine. J. Physiol. 244, 113–127 (1975).
Spencer, N., Walsh, M. & Smith, T. K. Does the guinea-pig ileum obey the 'law of the intestine'? J. Physiol. 517, 889–898 (1999).
Bennett, M. R. Rebound excitation of the smooth muscle cells of the guinea-pig taenia coli after stimulation of intramural inhibitory nerves. J. Physiol. 185, 124–131 (1966).
Bywater, R. A., Holman, M. E. & Taylor, G. S. Atropine-resistant depolarization in the guinea-pig small intestine. J. Physiol. 316, 369–378 (1981).
Benham, C. D., Bolton, T. B. & Lang, R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature 316, 345–347 (1985).
Inoue, R. & Isenberg, G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am. J. Physiol. 258, C1173–C1178 (1990).
Grady, E. F. et al. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 16, 6975–6986 (1996).
Cao, W. et al. Gq-linked NK(2) receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology 119, 51–61 (2000).
Lecci, A., Capriati, A., Altamura, M. & Maggi, C. A. Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human. Auton. Neurosci. 126–127, 232–249 (2006).
Mulè, F., Amato, A., Vannucchi, M. G., Faussone-Pellegrini, M. S. & Serio, R. Role of NK1 and NK2 receptors in mouse gastric mechanical activity. Br. J. Pharmacol. 147, 430–436 (2006).
Bult, H. et al. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345, 346–347 (1990).
Sanders, K. M. & Ward, S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am. J. Physiol. 262, G379–G392 (1992).
Koh, S. D. et al. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J. Biol. Chem. 276, 44338–44346 (2001).
Koh, S. D., Campbell, J. D., Carl, A. & Sanders, K. M. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J. Physiol. 489, 735–743 (1995).
Mutafova-Yambolieva, V. N. et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc. Natl Acad. Sci. USA 104, 16359–16364 (2007).
Hwang, S. J. et al. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140, 608–617 (2011).
Kurahashi, M. et al. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles. J. Physiol. 589, 697–710 (2011).
Gallego, D. et al. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J. Physiol. 590, 1943–1956 (2012).
Hwang, S. J. et al. P2Y1 Purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J. Physiol. 590, 1957–1972 (2012).
Goyal, R. K., Rattan, S. & Said, S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature 288, 378–380 (1980).
McConalogue, K. et al. Histochemical, pharmacological, biochemical and chromatographic evidence that pituitary adenylyl cyclase activating peptide is involved in inhibitory neurotransmission in the taenia of the guinea-pig caecum. J. Auton. Nerv. Syst. 50, 311–322 (1995).
Hagen, B. M., Bayguinov, O. & Sanders, K. M. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am. J. Physiol. Cell Physiol. 291, C375–C385 (2006).
Schulz, S. et al. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin. Cancer Res. 10, 8235–8242 (2004).
Kunze, W. A. & Furness, J. B. The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiol. 61, 117–42 (1999).
Szurszewski, J. H. in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.) 393–422 (Raven Press, New York, 1987).
Connor, J. A., Prosser, C. L. & Weems, W. A. A study of pace-maker activity in intestinal smooth muscle. J. Physiol. 240, 671–701 (1974).
Thuneberg, L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv. Anat. Embryol. Cell Biol. 71, 1–130 (1982).
Faussone Pellegrini, M. S., Cortesini, C. & Romagnoli, P. Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal's interstitial cells [Italian] Arch. Ital. Anat. Embriol. 82, 157–177 (1977).
Smith, T. K., Reed, J. B. & Sanders, K. M. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am. J. Physiol. 252, C215–C224 (1987).
Bauer, A. J., Publicover, N. G. & Sanders, K. M. Origin and spread of slow waves in canine gastric antral circular muscle. Am. J. Physiol. 249, G800–G806 (1985).
Hara, Y., Kubota, M. & Szurszewski, J. H. Electrophysiology of smooth muscle of the small intestine of some mammals. J. Physiol. 372, 501–520 (1986).
Langton, P., Ward, S. M., Carl, A., Norell, M. A. & Sanders, K. M. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc. Natl Acad. Sci USA 86, 7280–7284 (1989).
Zhu, M. H. et al. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 587, 4905–4918 (2009).
Ward, S. M., Burns, A. J., Torihashi, S. & Sanders, K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 480, 91–97 (1994).
Torihashi, S. et al. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 280, 97–111 (1995).
Huizinga, J. D. et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349 (1995).
Burns, A. J. Disorders of interstitial cells of Cajal. J. Pediatr. Gastroenterol. Nutr. 45 (Suppl. 2), S103–S106 (2007).
Farrugia, G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 20 (Suppl. 1), 54–63 (2008).
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Burns, A. J., Lomax, A. E., Torihashi, S., Sanders, K. M. & Ward, S. M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl Acad. Sci. USA 93, 12008–12013 (1996).
Burns, A. J., Herbert, T. M., Ward, S. M. & Sanders, K. M. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 290, 11–20 (1997).
Komuro, T., Seki, K. & Horiguchi, K. Ultrastructural characterization of the interstitial cells of Cajal. Arch. Histol. Cytol. 62, 295–316 (1999).
Komuro, T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J. Physiol. 576, 653–658 (2006).
Dickens, E. J., Hirst, G. D. & Tomita, T. Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 514, 515–531 (1999).
Kito, Y. & Suzuki, H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J. Physiol. 553, 803–818 (2003).
Cousins, H. M., Edwards, F. R., Hickey, H., Hill, C. E. & Hirst, G. D. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J. Physiol. 550, 829–844 (2003).
Thomsen, L. et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 4, 848–851 (1998).
Koh, S. D., Sanders, K. M. & Ward, S. M. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J. Physiol. (Lond.) 513, 203–213 (1998).
Zhu, M. H. et al. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 587, 4905–4918 (2009).
Ro, S. et al. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology 138, 1068–1078 (2010).
Hwang, S. J. et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 587, 4887–4904 (2009).
Imaizumi, M. & Hama, K. An electromicroscopic study on the interstitial cells of the gizzard in the love bird (Uronlonchu domestica). Z. Zellforsch. Mikrosk. Anat. 97, 351–357 (1969).
Daniel, E. E. & Posey-Daniel, V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am. J. Physiol. 246, G305–G315 (1984).
Cajal, S. R. Histologie du système nerveux de l'homme et des vertébrés. Vol. 2 891–942 (Maloine, Paris, 1911).
Chen, H. et al. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am. J. Physiol. Cell Physiol. 292, C497–C507 (2007).
Iino, S., Horiguchi, K., Nojyo, Y., Ward, S. M. & Sanders, K. M. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol. Motil. 21, 542–550 (2009).
Beckett, E. A., Takeda, Y., Yanase, H., Sanders, K. M. & Ward, S. M. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J. Comp. Neurol. 493, 193–206 (2005).
Ward, S. M. et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 20, 1393–1403 (2000).
Beckett, E. A., Horiguchi, K., Khoyi, M., Sanders, K. M. & Ward, S. M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J. Physiol. 543, 871–887 (2002).
Albertí, E. et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1499–G1510 (2007).
Huizinga, J. D. et al. Deficiency of intramuscular ICC increases fundic muscle excitability but does not impede nitrergic innervation. Am. J. Physiol. Gastr ointest. Liver Physiol. 294, G589–G594 (2008).
Sanders, K. M., Hwang, S. J. & Ward, S. M. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J. Physiol. 588, 4621–4639 (2010).
Zhu, M. H. et al. Muscarinic activation of Ca2+-activated Cl- current in interstitial cells of Cajal. J. Physiol. 589, 4565–4582 (2011).
Gabella, G. & Blundell, D. Gap junctions of the muscles of the small and large intestine. Cell Tissue Res. 219, 469–488 (1981).
Daniel, E. E. & Wang, Y. F. Gap junctions in intestinal smooth muscle and interstitial cells of Cajal. Microsc. Res. Tech. 47, 309–320 (1999).
Seki, K., Zhou, D. S. & Komuro, T. Immunohistochemical study of the c-kit expressing cells and connexin 43 in the guinea-pig digestive tract. J. Auton. Nerv. Syst. 68, 182–187 (1998).
Seki, K. & Komuro, T. Immunocytochemical demonstration of the gap junction proteins connexin 43 and connexin 45 in the musculature of the rat small intestine. Cell Tissue Res. 306, 417–422 (2001).
Li, Z., Zhou, Z. & Daniel, E. E. Expression of gap junction connexin 43 and connexin 43 mRNA in different regional tissues of intestine in dog. Am. J. Physiol. 265, G911–G916 (1993).
Vogalis, F. & Goyal, R. K. Activation of small conductance Ca2+-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J. Physiol. 502, 497–508 (1997).
Koh, S. D., Dick, G. M. & Sanders, K. M. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. 273, C2010–C2021 (1997).
Ishikawa, K., Komuro, T., Hirota, S. & Kitamura, Y. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res. 289, 137–143 (1997).
Horiguchi, K. & Komuro, T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/WV mouse small intestine. J. Auton. Nerv. Syst. 80, 142–147 (2000).
Mitsui, R. & Komuro, T. Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat. Embryol. 206, 453–460 (2003).
Klemm, M. F. & Lang, R. J. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin. Exp. Pharmacol. Physiol. 29, 18–25 (2002).
Vanderwinden, J. M. et al. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 310, 349–358 (2002).
Iino, S., Horiguchi, K., Horiguchi, S. & Nojyo, Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem. Cell. Biol. 131, 691–702 (2009).
Iino, S. & Nojyo, Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch. Histol. Cytol. 72, 107–115 (2009).
Laube, H. R., Duwe, J., Rutsch, W. & Konertz, W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J. Thorac. Cardiovasc. Surg. 120, 134–141 (2000).
Beamish, J. A., He, P., Kottke-Marchant, K. & Marchant, R. E. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng. Part B Rev. 16, 467–491 (2010).
Bitar, K. N. & Raghavan, S. Intestinal tissue engineering: current concepts and future vision of regenerative medicine in the gut. Neurogastroenterol. Motil. 24, 7–19 (2012).
Raghavan, S. et al. Successful implantation of physiologically functional bioengineered mouse internal anal sphincter. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G430–G439 (2010).
Raghavan, S. et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 141, 310–319 (2011).
Miano, J. M., Long, X. & Fujiwara, K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 292, C70–C81 (2007).
Wang, Z. et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428, 185–189 (2004).
Boettger, T. et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Invest. 119, 2634–2647 (2009).
Cordes, K. R. et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 (2009).
Park, C. et al. Serum response factor-dependent MicroRNAs regulate gastrointestinal smooth muscle cell phenotypes. Gastroenterology 141, 164–175 (2011).
Ji, R. et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 100, 1579–1588 (2007).
Lin, Y. et al. Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J. Biol. Chem. 284, 7903–7913 (2009).
Chen, J. et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler. Thromb. Vasc. Biol. 31, 368–375 (2011).
Torella, D. et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ. Res. 109, 880–893 (2011).
Xu, N., Papagiannakopoulos, T., Pan, G., Thomson, J. A. & Kosik, K. S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137, 647–658 (2009).
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Park, C. et al. MicroRNAs dynamically remodel gastrointestinal smooth muscle cells. PLoS ONE 6, e18628 (2011).
Dickson, L. & Finlayson, K. VPAC and PAC receptors: From ligands to function. Pharmacol. Ther. 121, 294–316 (2009).
Acknowledgements
The work of these authors and time spent on this Review was supported by grants from the NIDDK: R37 DK40569 (K. M. Sanders), P01 DK41315 (K. M. Sanders, S. D. Koh and S. M. Ward) and P20 GM103513 (S. Ro). The authors are grateful for discussions with Dr B. Perrino and his comments on the manuscript. We apologize if we have neglected the work of some investigators; research on gastrointestinal smooth muscle biology is bountiful and could not be reviewed exhaustively in this limited space.
Author information
Authors and Affiliations
Contributions
K. M. Sanders contributed to all aspects of this manuscript. S. D. Koh and S. M. Ward contributed to the discussion of content and reviewing/editing the manuscript. S. Ro contributed to writing and reviewing/editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sanders, K., Koh, S., Ro, S. et al. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9, 633–645 (2012). https://doi.org/10.1038/nrgastro.2012.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.168
This article is cited by
-
Total Flavonoids of Aurantii Fructus Immaturus Regulate miR-5100 to Improve Constipation by Targeting Fzd2 to Alleviate Calcium Balance and Autophagy in Interstitial Cells of Cajal
Molecular Neurobiology (2024)
-
The effectiveness of walking exercise on the bowel preparation before colonoscopy: a single blind randomized clinical trial study
BMC Gastroenterology (2023)
-
Contractility-induced self-organization of smooth muscle cells: from multilayer cell sheets to dynamic three-dimensional clusters
Communications Biology (2023)
-
Regenerative medicine: current research and perspective in pediatric surgery
Pediatric Surgery International (2023)
-
Amyloid-β mediates intestinal dysfunction and enteric neurons loss in Alzheimer's disease transgenic mouse
Cellular and Molecular Life Sciences (2023)