Key Points
-
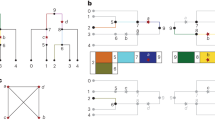
Mapping by admixture linkage disequilibrium (MALD) is a genetic strategy for discovering genes that underlie complex diseases. The method is based on differences in disease-gene frequency between the parental racial groups of admixed populations.
-
A MALD-based full-genome scan can be carried out using a few thousand markers that are able to differentiate, to a high degree, between chromosomal ancestries in relation to the parental popultions. This enables the discovery of regions that harbour genes associated with complex diseases.
-
Differences in the proportion of admixture for a particular chromosomal segment between cases and controls can implicate a region that is several centimorgans in size as being involved in a disease. This can also be done using cases only, by looking for differences in admixture proportions between specific locations and the rest of the genome in the same individual.
-
MALD-based genome scans are already possible in African-Americans, and are now underway. These studies are using a published set of MALD markers that are highly enriched for the ability to differentiate between chromosomal segments derived from African and European ancestors. The marker set will improve as frequency data accumulate from the HapMap project.
-
MALD scans in other groups (Latinos, Pacific Islanders and other admixed populations) will become possible in the near future as appropriate markers are mined from HapMap allele frequencies.
-
Proof of the efficacy of MALD awaits its successful application among African-Americans for potentially amenable diseases, such as prostate cancer, multiple sclerosis and end-stage renal disease. If these studies are successful, MALD could then be applied to other groups over the next few years.
Abstract
Mapping by admixture linkage disequilibrium (MALD) is a theoretically powerful, although unproven, approach to mapping genetic variants that are involved in human disease. MALD takes advantage of long-range haplotypes that are generated by gene flow among recently admixed ethnic groups, such as African-Americans and Latinos. Under ideal circumstances, MALD will have more power to detect some genetic variants than other types of genome-wide association study that are carried out among more ethnically homogeneous populations. It will also require 200–500 times fewer markers, providing a significant economic advantage. The MALD approach is now being applied, with results expected in the near future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Risch, N. & Merikangas, K. The future of genetic studies of complex human diseases. Science 273, 1516–1517 (1996).
Gibbs, R. A. et al. The International HapMap Project. Nature 426, 789–796 (2003).
Hirschhorn, J. N. & Daly, M. J. Genome-wide association studies for common diseases and complex traits. Nature Rev. Genet. 6, 95–108 (2005).
Wang, W. Y., Barratt, B. J., Clayton, D. G. & Todd, J. A. Genome-wide association studies: theoretical and practical concerns. Nature Rev. Genet. 6, 109–118 (2005).
Briscoe, D., Stephens, J. C. & O'Brien, S. J. Linkage disequilibrium in admixed populations: applications in gene mapping. J. Hered. 85, 59–63 (1994).
Stephens, J. C., Briscoe, D. & O'Brien, S. J. Mapping by admixture linkage disequilibrium in human populations: limits and guidelines. Am. J. Hum. Genet. 55, 809–824 (1994). This is an early description of the power of MALD for identifying disease genes by exploring models of gene discovery. This paper describes the characteristics of suitable populations and the consequences of admixture for linkage disequilibrium.
McKeigue, P. M. Mapping genes that underlie ethnic differences in disease risk: methods for detecting linkage in admixed populations, by conditioning on parental admixture. Am. J. Hum. Genet. 63, 241–251 (1998). This paper conceptualizes and describes the idea of gene mapping using admixture analysis based on chromosome segments that are derived from ancestral populations.
Darvasi, A. & Shifman, S. The beauty of admixture. Nature Genet. 37, 118–119 (2005).
Dean, M. et al. Polymorphic admixture typing in human ethnic populations. Am. J. Hum. Genet. 55, 788–808 (1994).
Smith, M. W. et al. Markers for mapping by admixture linkage disequilibrium in African American and Hispanic populations. Am. J. Hum. Genet. 69, 1080–1094 (2001).
Smith, M. W. et al. A high-density admixture map for disease gene discovery in African Americans. Am. J. Hum. Genet. 74, 1001–1013 (2004). A genome-wide set of markers for MALD-based gene discovery in African-Americans is described in this paper. An average of 6 generations since admixture and average European chromosomal segments with block sizes of 11 cM are estimated.
Montana, G. & Pritchard, J. K. Statistical tests for admixture mapping with case–control and cases-only data. Am. J. Hum. Genet. 75, 771–789 (2004). The authors describe the use of the program STRUCTURE and MALDsoft for admixture mapping with simulated data. They suggest that a large set of random SNPs can be used to discover disease genes nearly as well as a much smaller set of markers that are enriched for MALD-based gene discovery.
Patterson, N. et al. Methods for high-density admixture mapping of disease genes. Am. J. Hum. Genet. 74, 979–1000 (2004). This paper describes a rapid gene-mapping algorithm (ANCESTRYMAP) for MALD that uses MCMC and hidden Markov chain methodologies that are capable of whole-genome admixture scans. It extensively models the sample sizes that are necessary for gene discovery using MALD.
Seldin, M. F. et al. Putative ancestral origins of chromosomal segments in individual African Americans: implications for admixture mapping. Genome Res. 14, 1076–1084 (2004).
Zhang, C., Chen, K., Seldin, M. F. & Li, H. A hidden Markov modeling approach for admixture mapping based on case–control data. Genet. Epidemiol. 27, 225–239 (2004).
Zhu, X., Cooper, R. S. & Elston, R. C. Linkage analysis of a complex disease through use of admixed populations. Am. J. Hum. Genet. 74, 1136–1153 (2004).
Chakraborty, R. & Weiss, K. M. Admixture as a tool for finding linked genes and detecting that difference from allelic association between loci. Proc. Natl Acad. Sci. USA 85, 9119–9123 (1988). A classic reference that describes admixture and its potential use in finding traits of interest.
Hoggart, C. J. et al. Control of confounding of genetic associations in stratified populations. Am. J. Hum. Genet. 72, 1492–1504 (2003).
Hoggart, C. J., Shriver, M. D., Kittles, R. A., Clayton, D. G. & McKeigue, P. M. Design and analysis of admixture mapping studies. Am. J. Hum. Genet. 74, 965–978 (2004). This article describes a methodology for admixture analysis and makes sample-size estimates for MALD-based gene discovery.
Parra, E. J. et al. Estimating African American admixture proportions by use of population-specific alleles. Am. J. Hum. Genet. 63, 1839–1851 (1998).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data. Linked loci and correlated allele frequencies. Genetics 164, 1567–1587 (2003).
Halder, I. & Shriver, M. D. Measuring and using admixture to study the genetics of complex disease. Hum. Genomics 1, 52–62 (2003).
Wang, J. Maximum-likelihood estimation of admixture proportions from genetic data. Genetics 164, 747–765 (2003).
Reiner, A. P. et al. Population structure, admixture, and aging-related phenotypes in African American adults: the cardiovascular health study. Am. J. Hum. Genet. 76, 463–477 (2005).
Madrigal, L. et al. Ethnicity, gene flow, and population subdivision in Limon, Costa Rica. Am. J. Phys. Anthropol. 114, 99–108 (2001).
Bertoni, B., Budowle, B., Sans, M., Barton, S. A. & Chakraborty, R. Admixture in Hispanics: distribution of ancestral population contributions in the continental United States. Hum. Biol. 75, 1–11 (2003).
Bonilla, C., Shriver, M. D., Parra, E. J., Jones, A. & Fernandez, J. R. Ancestral proportions and their association with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Hum. Genet. (2004).
Collins-Schramm, H. E. et al. Mexican American ancestry-informative markers: examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum. Genet. 114, 263–271 (2004).
Parra, E. J. et al. Relation of type 2 diabetes to individual admixture and candidate gene polymorphisms in the Hispanic American population of San Luis Valley, Colorado. J. Med. Genet. 41, e116 (2004).
Rousham, E. K. & Gracey, M. Factors affecting birthweight of rural Australian Aborigines. Ann. Hum. Biol. 29, 363–372 (2002).
Grandinetti, A. et al. Relationship between plasma glucose concentrations and Native Hawaiian ancestry: the Native Hawaiian Health Research Project. Int. J. Obes. Relat. Metab. Disord. 26, 778–782 (2002).
Pfaff, C. L. et al. Population structure in admixed populations: effect of admixture dynamics on the pattern of linkage disequilibrium. Am. J. Hum. Genet. 68, 198–207 (2001).
Lautenberger, J. A., Stephens, J. C., O'Brien, S. J. & Smith, M. W. Significant admixture linkage disequilibrium across 30 cM around the FY locus in African Americans. Am. J. Hum. Genet. 66, 969–978 (2000).
Zhu, X. et al. Admixture mapping for hypertension loci with genome-scan markers. Nature Genet. 37, 177–181 (2005).
Hinds, D. A. et al. Whole-genome patterns of common DNA variation in three human populations. Science 307, 1072–1079 (2005).
McKeigue, P. M. Prospects for admixture mapping of complex traits. Am. J. Hum. Genet. 76, 1–7 (2004).
Mountain, J. L. & Risch, N. Assessing genetic contributions to phenotypic differences among 'racial' and 'ethnic' groups. Nature Genet. 36, S48–S53 (2004).
Schwartz, R. S. Racial profiling in medical research. N. Engl. J. Med. 344, 1392–1393 (2001).
Braun, L. Race, ethnicity, and health: can genetics explain disparities? Perspect. Biol. Med. 45, 159–174 (2002).
Pearce, N., Foliaki, S., Sporle, A. & Cunningham, C. Genetics, race, ethnicity, and health. BMJ 328, 1070–1072 (2004).
Risch, N., Burchard, E., Ziv, E. & Tang, H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 3, 1–12 (2002).
Burchard, E. G. et al. The importance of race and ethnic background in biomedical research and clinical practice. N. Engl. J. Med. 348, 1170–1175 (2003).
Bamshad, M., Wooding, S., Salisbury, B. A. & Stephens, J. C. Deconstructing the relationship between genetics and race. Nature Rev. Genet. 5, 598–609 (2004).
Taylor, A. L. et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 351, 2049–2057 (2004).
McEliece, R. The Theory of Information and Coding. Encyclopaedia of Math and its Applications Vol. 3 (Addison Wesley, 1977).
Reich, D. & Patterson, N. Pitfalls and prospects for admixture mapping. Philos. Trans. R. Soc. B (in the press).
Thomas, D. L. et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 284, 450–456 (2000).
Tess, B. H., Rodrigues, L. C., Newell, M. L., Dunn, D. T. & Lago, T. D. Breastfeeding, genetic, obstetric and other risk factors associated with mother-to-child transmission of HIV-1 in Sao Paulo State, Brazil. Sao Paulo collaborative study for vertical transmission of HIV-1. Aids 12, 513–520 (1998).
Hogancamp, W. E., Rodriguez, M. & Weinshenker, B. G. The epidemiology of multiple sclerosis. Mayo Clin. Proc. 72, 871–878 (1997).
Ruo, B., Capra, A. M., Jensvold, N. G. & Go, A. S. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J. Am. Coll. Cardiol. 43, 429–435 (2004).
Gupta, V. et al. Racial differences in thoracic aorta atherosclerosis among ischemic stroke patients. Stroke 34, 408–412 (2003).
Bohannon, A. D. Osteoporosis and African American women. J. Womens Health Gend. Based Med. 8, 609–615 (1999).
Finkelstein, J. S. et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J. Clin. Endocrinol. Metab. 87, 3057–3067 (2002).
Bastian, H. M. et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 11, 152–160 (2002).
Davey Smith, G., Neaton, J. D., Wentworth, D., Stamler, R. & Stamler, J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. Lancet 351, 934–939 (1998).
Demirovic, J. et al. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann. Epidemiol. 13, 472–478 (2003).
Harper, M. A. et al. Racial disparity in pregnancy-related mortality following a live birth outcome. Ann. Epidemiol. 14, 274–279 (2004).
Kopp, J. B. & Winkler, C. HIV-associated nephropathy in African Americans. Kidney Int. S43–S49 (2003).
Songer, T. J. & Zimmet, P. Z. Epidemiology of type II diabetes: an international perspective. Pharmacoeconomics 8 (Suppl. 1), 1–11 (1995).
Klag, M. J. et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277, 1293–1298 (1997).
Kissela, B. et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 35, 426–431 (2004).
Wong, T. Y. et al. Racial differences in the prevalence of hypertensive retinopathy. Hypertension 41, 1086–1091 (2003).
McGinnis, K. A. et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am. J. Public Health 93, 1728–1733 (2003).
Hodge, A. M. & Zimmet, P. Z. The epidemiology of obesity. Baillieres Clin. Endocrinol. Metab. 8, 577–599 (1994).
Molokhia, M. & McKeigue, P. Risk for rheumatic disease in relation to ethnicity and admixture. Arthritis Res. 2, 115–125 (2000).
Reveille, J. D. Ethnicity and race and systemic sclerosis: how it affects susceptibility, severity, antibody genetics, and clinical manifestations. Curr. Rheumatol. Rep. 5, 160–167 (2003).
Wright, N. M., Papadea, N., Veldhuis, J. D. & Bell, N. H. Growth hormone secretion and bone mineral density in prepubertal black and white boys. Calcif. Tissue Int. 70, 146–152 (2002).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1
(PDF 160 kb)
Supplementary information S2
(PDF 178 kb)
Supplementary information S3
(PDF 77 kb)
Related links
Related links
DATABASES
Entrez
FURTHER INFORMATION
Family Linkage Mapping resources at the Marshfield Center for Medical Genetics
Glossary
- LINKAGE MAPPING
-
A method for localizing genes that is based on the co-inheritance of genetic markers and phenotypes in families over several generations.
- ASSOCIATION STUDIES
-
A gene-discovery strategy that compares cases with controls to assess the contribution of genetic variants to phenotypes in specific populations.
- HAPLOTYPE
-
The sequence of a single chromosome, summarized as a unique combination of known polymorphic sites.
- ADMIXTURE
-
The formation of a new population by interbreeding between individuals from genetically divergent parental populations, and subsequently by interbreeding between their offspring.
- ADMIXTURE LINKAGE DISEQUILIBRIUM
-
The non-random association of genetic variants due to admixture that decays rapidly (in a few generations) between unlinked genes and more slowly between linked ones.
- FIXATION
-
Fixation occurs when a specific allele at a locus is found exclusively in one population but in another, an alternative allele is exclusively present.
- CONTINGENC Y ANALYSIS
-
A chi-squared analysis of the numbers of observations to test for differences between categories in a data table.
- LEAST-SQUARED APPROACH
-
A statistical estimation technique that estimates parameters on the basis of minimizing the square of the differences between a model and the observations.
- MAXIMUM LIKELIHOOD APPROACH
-
A method for estimating parameter values in a model that have the highest probability of explaining the data observed.
- BAYESIAN APPROACH
-
A statistical methodology that takes prior knowledge into account.
- PANMIXIA
-
The process by which individuals in a population choose each other as mates with equal likelihood.
- DUFFY BLOOD GROUP
-
Encoded by the FY gene, this is an antigen expressed on red blood cells that is a scavenger receptor for chemokines and also serves as a receptor for the malarial parasite, Plasmodium vivax.
- SHANNON INFORMATION CONTENT
-
A measure that is used to quantify the informativeness of a marker or set of markers for determining the ancestral state of a chromosomal segment or locus.
- HIDDEN MARKOV MODEL
-
(HMM). A statistical model of a sequence of events for which the probability of an event occurring depends on previous and subsequent events occurring. It is useful in admixture mapping as a complex and interdependent model can be calculated to fit the segmental nature of admixed chromosomes.
- MARKOV CHAIN MONTE CARLO
-
(MCMC). The distributions underlying the hidden Markov model are extremely complex, making their direct estimation a huge task. This is simplified in MCMC analysis by generating averages of the expectations from the underlying distributions to model and analyse the results of admixture mapping.
- PARAMETRIC TESTS
-
Statistical tests which use models that make assumptions about the distributions of sample values and parameters.
- NON-PARAMETRIC TESTS
-
Statistical procedures that are not based on models or assumptions pertaining to the distribution of the variable.
- PERMUTATION TESTING
-
An approach in which the actual data are randomized many times to generate a distribution of outcomes, so that the fraction of observations with values that are more extreme than the outcome that is observed with the real data reflects the statistical significance.
Rights and permissions
About this article
Cite this article
Smith, M., O'Brien, S. Mapping by admixture linkage disequilibrium: advances, limitations and guidelines. Nat Rev Genet 6, 623–632 (2005). https://doi.org/10.1038/nrg1657
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg1657
This article is cited by
-
Admixture mapping of anthropometric traits in the Black Women’s Health Study: evidence of a shared African ancestry component with birth weight and type 2 diabetes
Journal of Human Genetics (2022)
-
Apparent latent structure within the UK Biobank sample has implications for epidemiological analysis
Nature Communications (2019)
-
Genomics of disease risk in globally diverse populations
Nature Reviews Genetics (2019)
-
Inference of multiple-wave population admixture by modeling decay of linkage disequilibrium with polynomial functions
Heredity (2017)
-
Mapping the genomic architecture of adaptive traits with interspecific introgressive origin: a coalescent-based approach
BMC Genomics (2016)