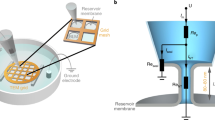
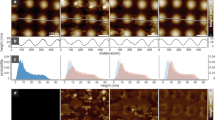
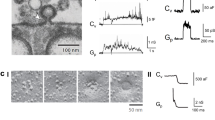
Abstract
The process of membrane fission is fundamental to diverse cellular processes such as nutrient uptake, synaptic transmission and organelle biogenesis, and it involves the localized application of curvature stress to a tubular membrane intermediate, forcing it to undergo scission. Alternative techniques for creating such substrates necessitate the use of micromanipulators or sophisticated optical traps and require a high level of technical expertise. We present a facile method to generate an array of membrane tubes supported on a passivated glass coverslip, which we refer to as supported membrane tubes (SMrTs). SMrT templates are formed upon hydration of a dry lipid mix in physiological buffer and subsequent flow-induced extrusion of the lipid reservoir into long membrane tubes with variable dimensions. Following surface passivation of coverslips, these templates can be formed from a variety of lipids, with as little as 1–2 nmol of lipid in a matter of 2 h, and can be used in membrane-curvature-sensitive fission assays.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koenig, J., Saito, K. & Ikeda, K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J. Cell Biol. 96, 1517–1522 (1983).
van der Bliek, A.M. et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122, 553–563 (1993).
Praefcke, G.J.K. & McMahon, H.T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 (2004).
Schmid, S.L. & Frolov, V.A. Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 27, 79–105 (2011).
Ferguson, S.M. & De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75–88 (2012).
Miwako, I., Schröter, T. & Schmid, S.L. Clathrin- and dynamin-dependent coated vesicle formation from isolated plasma membranes. Traffic 4, 376–389 (2003).
Takei, K. et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131–141 (1998).
Kinuta, M. et al. Phosphatidylinositol 4,5-bisphosphate stimulates vesicle formation from liposomes by brain cytosol. Proc. Natl. Acad. Sci. USA 99, 2842–2847 (2002).
Boucrot, E. et al. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149, 124–136 (2012).
Pucadyil, T.J. & Schmid, S.L. Supported bilayers with excess membrane reservoir: a template for reconstituting membrane budding and fission. Biophys. J. 99, 517–525 (2010).
Neumann, S., Pucadyil, T.J. & Schmid, S.L. Analyzing membrane remodeling and fission using supported bilayers with excess membrane reservoir. Nat. Protoc. 8, 213–222 (2013).
Pucadyil, T.J. & Schmid, S.L. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135, 1263–1275 (2008).
Morlot, S. et al. Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction. Cell 151, 619–629 (2012).
Bashkirov, P.V. et al. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 135, 1276–1286 (2008).
Roux, A. et al. Membrane curvature controls dynamin polymerization. Proc. Natl. Acad. Sci. USA 107, 4141–4146 (2010).
Shnyrova, A.V. et al. Geometric catalysis of membrane fission driven by flexible dynamin rings. Science 339, 1433–1436 (2013).
Dar, S., Kamerkar, S.C. & Pucadyil, T.J. A high-throughput platform for real-time analysis of membrane fission reactions reveals dynamin function. Nat. Cell Biol. 17, 1588–1596 (2015).
Holkar, S.S., Kamerkar, S.C. & Pucadyil, T.J. Spatial control of epsin-induced clathrin assembly by membrane curvature. J. Biol. Chem. 290, 14267–14276 (2015).
Pucadyil, T.J. & Holkar, S.S. Comparative analysis of adaptor-mediated clathrin assembly reveals general principles for adaptor clustering. Mol. Biol. Cell 27, 3156–3163 (2016).
Hsieh, W.-T. et al. Curvature sorting of peripheral proteins on solid-supported wavy membranes. Langmuir 28, 12838–12843 (2012).
Jung, H., Robison, A.D. & Cremer, P.S. Detecting proteinligand binding on supported bilayers by local pH modulation. J. Am. Chem. Soc. 131, 1006–1014 (2009).
Kunding, A.H., Mortensen, M.W., Christensen, S.M. & Stamou, D. A fluorescence-based technique to construct size distributions from single-object measurements: application to the extrusion of lipid vesicles. Biophys. J. 95, 1176–1188 (2008).
Bassereau, P., Sorre, B. & Lévy, A. Bending lipid membranes: experiments after W. Helfrich's model. Adv. Colloid Interface Sci. 208, 47–57 (2014).
Frolov, V.A., Lizunov, V.A., Dunina-Barkovskaya, A.Y., Samsonov, A.V. & Zimmerberg, J. Shape bistability of a membrane neck: a toggle switch to control vesicle content release. Proc. Natl. Acad. Sci. USA 100, 8698–8703 (2003).
Koster, G., VanDuijn, M., Hofs, B. & Dogterom, M. Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc. Natl. Acad. Sci. USA 100, 15583–15588 (2003).
Baumgart, T., Capraro, B.R., Zhu, C. & Das, S.L. Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem. 62, 483–506 (2011).
Sorre, B. et al. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA 109, 173–178 (2012).
Roux, A. et al. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc. Natl. Acad. Sci. USA 99, 5394–5399 (2002).
Leduc, C. et al. Cooperative extraction of membrane nanotubes by molecular motors. Proc. Natl. Acad. Sci. USA 101, 17096–17101 (2004).
Roux, A., Uyhazi, K., Frost, A. & De Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 (2006).
Rossier, O., Cuvelier, D., Borghi, N., Puech, P.H. & Derényi, I. Giant vesicles under flows: extrusion and retraction of tubes. Langmuir 19, 575–584 (2003).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Piehler, J., Brecht, A., Valiokas, R., Liedberg, B. & Gauglitz, G. A high-density poly(ethylene glycol) polymer brush for immobilization on glass-type surfaces. Biosens. Bioelectron. 15, 473–481 (2000).
Bernazzani, L. et al. On the interaction of sodium dodecyl sulfate with oligomers of poly(ethylene glycol) in aqueous solution. J. Phys. Chem. B 108, 8960–8969 (2004).
Acknowledgements
S.D. and S.C.K. acknowledge the Council for Scientific and Industrial Research (CSIR) for fellowships. T.J.P. is an Intermediate Fellow of the Wellcome Trust-DBT India Alliance and thanks the Alliance and IISER Pune for funding.
Author information
Authors and Affiliations
Contributions
S.D., S.C.K. and T.J.P. developed the method. S.D. performed all experiments with dynamin. S.C.K. performed flow cell calibration. S.D., S.C.K. and T.J.P analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
41596_2017_BFnprot2016173_MOESM30_ESM.avi
A time-lapse sequence showing how flow rates can be estimated. The first 2 s of the video shows fluorescent liposomes moving in solution, following which the pump is turned on at a fine dial setting of 8. (AVI 2023 kb)
41596_2017_BFnprot2016173_MOESM31_ESM.avi
A time-lapse sequence showing how the lipid at the source is extruded into membrane tubes in response to buffer flow. The first 30 s of the video is acquired at a low flow rate to visualize tubules and buds formed at the source. These structures are subsequently extruded into membrane tubes upon increasing the flow rate. (AVI 3302 kb)
41596_2017_BFnprot2016173_MOESM32_ESM.avi
A time-lapse sequence showing the effect of flowing 0.5 μM dynamin in the presence of 1 mM GTP and 1 mM MgCl2 in HKS supplemented with oxygen scavengers into SMrT templates. (AVI 2752 kb)
41596_2017_BFnprot2016173_MOESM33_ESM.avi
A time-lapse sequence showing the effect of flowing 1 mM GTP and 1 mM MgCl2 in HKS into preassembled dynamin on SMrT templates. (AVI 1832 kb)
Rights and permissions
About this article
Cite this article
Dar, S., Kamerkar, S. & Pucadyil, T. Use of the supported membrane tube assay system for real-time analysis of membrane fission reactions. Nat Protoc 12, 390–400 (2017). https://doi.org/10.1038/nprot.2016.173
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.173
This article is cited by
-
The HEAT repeat protein HPO-27 is a lysosome fission factor
Nature (2024)
-
Metal-Binding Propensity in the Mitochondrial Dynamin-Related Protein 1
The Journal of Membrane Biology (2022)
-
Function and regulation of the divisome for mitochondrial fission
Nature (2021)
-
Mycobacterial dynamin-like protein IniA mediates membrane fission
Nature Communications (2019)
-
ATP-dependent membrane remodeling links EHD1 functions to endocytic recycling
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.