Abstract
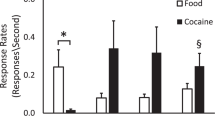
Dopamine neurotransmission is associated with high susceptibility to cocaine abuse. Positron emission tomography was used in 12 rhesus macaques to determine if dopamine D2 receptor availability was associated with the rate of cocaine reinforcement, and to study changes in brain dopaminergic function during maintenance of and abstinence from cocaine. Baseline D2 receptor availability was negatively correlated with rates of cocaine self-administration. D2 receptor availability decreased by 15–20% within 1 week of initiating self-administration and remained reduced by ∼20% during 1 year of exposure. Long-term reductions in D2 receptor availability were observed, with decreases persisting for up to 1 year of abstinence in some monkeys. These data provide evidence for a predisposition to self-administer cocaine based on D2 receptor availability, and demonstrate that the brain dopamine system responds rapidly following cocaine exposure. Individual differences in the rate of recovery of D2 receptor function during abstinence were noted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Neuroscience of Psychoactive Substance Use and Dependence. (World Health Organization, Geneva, Switzerland, 2004).
2003 National Survey on Drug Use and Health (NSDUH). (US Department of Health and Human Services, National Institute on Drug Abuse, Rockville, Maryland, 2004).
O'Brien, M.S. & Anthony, J.C. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology 30, 1006–1018 (2005).
Emergency Department Trends From The Drug Abuse Warning Network: Final Estimates 1994–2001. (US Department of Health and Human Services, National Institute on Drug Abuse, Rockville, Maryland, 2002).
Mello, N.K. & Negus, S.S. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14, 375–424 (1996).
Leshner, A.I. Vulnerability to addiction: new research opportunities. Am. J. Med. Genet. 96, 590–591 (2000).
Leshner, A.I. Addiction is a brain disease, and it matters. Science 278, 45–47 (1997).
Griffith, R.R., Bigelow, G.E. & Henningfield, J.E. Similarities in animal and human drug-taking behavior. in Advances in Substance Abuse Vol. 1 (ed. Mello, N.K.) 1–90 (JAI Press, Greenwich, Connecticut, 1980).
Spealman, R.D. & Goldberg, S.R. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu. Rev. Pharmacol. Toxicol. 18, 313–339 (1978).
Woolverton, W.L. & Nader, M.A. Experimental evaluation of the reinforcing effects of drugs. in Testing and Evaluation of Drugs of Abuse (eds. Adler, M.W. & Cowan, A.) 165–192 (Wiley-Liss, New York, 1990).
Reith, M.E., Sershen, H. & Lajtha, A. Saturable [3H]cocaine binding in central nervous system of mouse. Life Sci. 27, 1055–1062 (1980).
Madras, B.K., Fahey, M.A., Bergman, J., Canfield, D.R. & Spealman, R.D. Effects of cocaine and related drugs in nonhuman primates, I: [3H]cocaine binding sites in caudate-putamen. J. Pharmacol. Exp. Ther. 251, 131–141 (1989).
Blum, K., Cull, J.C., Braverman, E.R. & Comings, D.E. Reward deficiency syndrome. Am. Sci. 84, 132–145 (1996).
Nader, M.A. & Czoty, P.W. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am. J. Psychiatry 162, 1473–1482 (2005).
Volkow, N.D. et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am. J. Psychiatry 156, 1440–1443 (1999).
Morgan, D. et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 5, 169–174 (2002).
Volkow, N.D. et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14, 169–177 (1993).
Martinez, D. et al. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29, 1190–1202 (2004).
Dackis, C. & O'Brien, C. Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 8, 1431–1436 (2005).
Mach, R.H. et al. Comparison of two fluorine-18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptor: in vitro binding studies and positron emission tomography. Synapse 24, 322–333 (1996).
Mach, R.H. et al. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol. Biochem. Behav. 57, 477–486 (1997).
Dewey, S.L. et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J. Neurosci. 12, 3773–3780 (1992).
Nader, M.A. et al. PET imaging of dopamine D2 receptors with [18F] fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuropsychopharmacology 21, 589–596 (1999).
Logan, J. et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 10, 740–747 (1990).
Hietala, J., Kuoppamaki, M., Nagren, K., Lehikoinen, P. & Syvalahti, E. Effects of lorazepam administration on striatal dopamine D2 receptor binding characteristics in man – a positron emission tomography study. Psychopharmacology (Berl.) 132, 361–365 (1997).
Zernig, G., Wakonigg, G., Madlung, E., Haring, C. & Saria, A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology (Berl.) 171, 349–351 (2004).
Laruelle, M. et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17, 162–174 (1997).
Kalivas, P.W. & Duffy, P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol. Psychiatry 25, 913–928 (1989).
Piazza, P.V. & Le Moal, M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 19, 67–74 (1998).
Goeders, N.E. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 301, 785–789 (2002).
Volkow, N.D. & Folwer, J.S. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex 10, 318–325 (2000).
Bradberry, C.W. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J. Neurosci. 20, 7109–7115 (2000).
Moore, R., Vinsant, S.L., Nader, M.A., Porrino, L.J. & Friedman, D.P. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse 30, 88–96 (1998).
Nader, M.A. et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 27, 35–46 (2002).
Robinson, T.E. & Berridge, K.C. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 18, 247–291 (1993).
Piazza, P.V., Deminiere, J.-M., Le Moal, M. & Simon, H. Factors that predict individual vulnerability to amphetamine self-administration. Science 245, 1511–1513 (1989).
Koob, G.F. & Le Moal, M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat. Neurosci. 8, 1442–1444 (2005).
Grigson, P.S. & Twining, R.C. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav. Neurosci. 116, 321–333 (2002).
Ahmed, S.H. & Koob, G.F. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282, 298–300 (1998).
Calabresi, P., De Murtas, M. & Bernardi, G. The neostriatum beyond the motor function: experimental and clinical evidence. Neuroscience 78, 39–60 (1997).
Piazza, P.V., Deroche-Gamonent, V., Rouge-Pont, F. & Le Moal, M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci. 20, 4226–4232 (2000).
Volkow, N.D. et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am. J. Psychiatry 147, 719–724 (1990).
Czoty, P.W., Gage, H.D. & Nader, M.A. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse 58, 215–219 (2005).
Dewey, S.L. et al. Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J. Neurosci. 15, 821–829 (1995).
Mach, R.H. et al. The use of [18F]4-fluorobenzyl iodide (FBI) in PET radiotracer synthesis: model alkylation studies and its application in the design of dopamine D1 and D2 receptor-based imaging agents. Nucl. Med. Biol. 20, 777–794 (1993).
Mach, R.H. et al. 18F-labeled benzamides for studying the dopamine D2 receptor with positron emission tomography. J. Med. Chem. 36, 3707–3720 (1993).
Mintun, M.A., Raichle, M.E., Kilbourn, M.R., Wooten, G.F. & Welch, M.J. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann. Neurol. 15, 217–227 (1984).
Acknowledgements
We thank P.W. Czoty, M.L. Banks and K.A. Grant for comments on the manuscript, and C. Hubbard, T. Morton and R. Kuhner for technical assistance. This research was supported by the National Institute on Drug Abuse (grants DA 08468 and DA 14637).
Author information
Authors and Affiliations
Contributions
M.A.N., D.M., S.H.N and T.L.C. contributed to the cocaine self-administration studies; H.D.G., N.B., R.E. and R.H.M. contributed to the PET imaging studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nader, M., Morgan, D., Gage, H. et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci 9, 1050–1056 (2006). https://doi.org/10.1038/nn1737
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1737
This article is cited by
-
Acquisition of cocaine reinforcement using fixed-ratio and concurrent choice schedules in socially housed female and male monkeys
Psychopharmacology (2024)
-
A non-human primate model of cocaine addiction: interpreting associations with increased vs. decreased dopamine function
Neuropsychopharmacology (2023)
-
PET imaging of kappa opioid receptors and receptor expression quantified in neuron-derived extracellular vesicles in socially housed female and male cynomolgus macaques
Neuropsychopharmacology (2023)
-
PET imaging of dopamine transporters and D2/D3 receptors in female monkeys: effects of chronic cocaine self-administration
Neuropsychopharmacology (2023)
-
Cell-type specific synaptic plasticity in dorsal striatum is associated with punishment-resistance compulsive-like cocaine self-administration in mice
Neuropsychopharmacology (2023)