Abstract
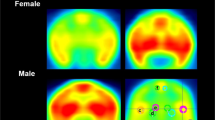
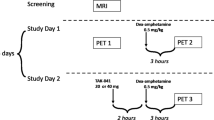
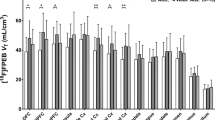
Brain imaging studies using positron emission tomography (PET) have shown that long-term cocaine use is associated with lower levels of dopamine (DA) D2/D3 receptors (D2/D3R); less consistent are the effects on DA transporter (DAT) availability. However, most studies have been conducted in male subjects (humans, monkeys, rodents). In this study, we used PET imaging in nine drug-naïve female cynomolgus monkeys to determine if baseline measures of DAT, with [18F]FECNT, and D2/D3R availability, with [11C]raclopride, in the caudate nucleus, putamen and ventral striatum were associated with rates of cocaine self-administration and if these measures changed during long-term (~13 months) cocaine self-administration and following time-off (3–9 months) from cocaine. Cocaine (0.2 mg/kg/injection) and 1.0 g food pellets were available under a multiple fixed-interval (FI) 3-min schedule of reinforcement. In contrast to what has been observed in male monkeys, baseline D2/D3R availability was positively correlated with rates of cocaine self-administration only during the first week of exposure; DAT availability did not correlate with cocaine self-administration. D2/D3R availability decreased ~20% following cumulative intakes of 100 and 1000 mg/kg cocaine; DAT availability did not significantly change. These reductions in D2/D3R availability did not recover over 9 months of time-off from cocaine. To determine if these reductions were reversible, three monkeys were implanted with osmotic pumps that delivered raclopride for 30 days. We found that chronic treatment with the D2/D3R antagonist raclopride increased D2/D3R availability in the ventral striatum but not in the other regions when compared to baseline levels. Over 13 months of self-administration, tolerance did not develop to the rate-decreasing effects of self-administered cocaine on food-reinforced responding, but number of injections and cocaine intake significantly increased over the 13 months. These data extend previous findings to female monkeys and suggest sex differences in the relationship between D2/D3R availability related to vulnerability and long-term cocaine use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Crime. UNODC World Drug Report. https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2022.html. 2022.
Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218:108350.
SAMHSA, Key substance use and mental health indicators in the united states: Results from the 2021 national survey on drug use and health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57), Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2022. Retrieved from https://www.samhsa.gov/data/.
O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31.
Elkashef A, Biswas J, Acri JB, Vocci F. Biotechnology and the treatment of addictive disorders: new opportunities. BioDrugs. 2007;21:259–67.
Haile CN, Mahoney JJ 3rd, Newton TF, De La Garza R 2nd. Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharm Ther. 2012;134:260–77.
Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX. New drugs, old targets: tweaking the dopamine system to treat psychostimulant use disorders. Annu Rev Pharm Toxicol. 2021;61:609–28.
Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22:61–74.
O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30:1006–18.
Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–55.
Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–6.
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharm Exp Ther. 1999;288:14–20.
Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008.
Alvanzo AA, Wand GS, Kuwabara H, Wong DF, Xu X, McCaul ME. Family history of alcoholism is related to increased D(2) /D(3) receptor binding potential: a marker of resilience or risk? Addict Biol. 2017;22:218–28.
Spitta G, Gleich T, Zacharias K, Butler O, Buchert R, Gallinat J. Extrastriatal Dopamine D2/3 Receptor Availability in Alcohol Use Disorder and Individuals at High Risk. Neuropsychobiology. 2022;81:215–24.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–3.
Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70.
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–41.
Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse. 2005;58:215–9.
Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, et al. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012;72:414–21.
Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharm Exp Ther. 1994;271:1678–85.
Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–807.
Wang GJ, Volkow ND, Fowler JS, Fischman M, Foltin R, Abumrad NN, et al. Cocaine abusers do not show loss of dopamine transporters with age. Life Sci. 1997;61:1059–65.
Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, et al. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–4.
Czoty PW, Gage HD, Nader SH, Reboussin BA, Bounds M, Nader MA. PET imaging of dopamine D2 receptor and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J Addict Med. 2007;1:33–9.
Goodman MM, Kung MP, Kabalka GW, Kung HF, Switzer R. Synthesis and characterization of radioiodinated N-(3-iodopropen-1-yl)-2 beta-carbomethoxy-3 beta-(4-chlorophenyl)tropanes: potential dopamine reuptake site imaging agents. J Med Chem. 1994;37:1535–42.
Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, et al. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27:1–12.
Appt SE. Usefulness of the monkey model to investigate the role soy in postmenopausal women’s health. ILAR J. 2004;45:200–11.
Kromrey SA, Czoty PW, Nader MA. Relationship between estradiol and progesterone concentrations and cognitive performance in normally cycling female cynomolgus monkeys. Horm Behav. 2015;72:12–9.
Howell LL, Wilcox KM. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology. 2002;163:352–61.
Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, et al. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–10.
Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedstrom CG, et al. Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci USA 1985;82:3863–7.
Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34:548–54.
Nader MA, Grant KA, Gage HD, Ehrenkaufer RL, Kaplan JR, Mach RH. PET imaging of dopamine D2 receptors with [18F]fluoroclebopride in monkeys: effects of isoflurane- and ketamine-induced anesthesia. Neuropsychopharmacology. 1999;21:589–96.
Martinez D, Slifstein M, Matuskey D, Nabulsi N, Zheng MQ, Lin SF, et al. Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study. Neuropsychopharmacology. 2019;44:1720–7.
Matuskey D, Dias M, Naganawa M, Pittman B, Henry S, Li S, et al. Social status and demographic effects of the kappa opioid receptor: a PET imaging study with a novel agonist radiotracer in healthy volunteers. Neuropsychopharmacology. 2019;44:1714–9.
Johnson BN, Kumar A, Su Y, Singh S, Sai KKS, Nader SH, et al. PET imaging of kappa opioid receptors and receptor expression quantified in neuron-derived extracellular vesicles in socially housed female and male cynomolgus macaques. Neuropsychopharmacology. 2023;48:410–7.
Karkhanis A, Holleran KM, Jones SR. Dynorphin/Kappa opioid receptor signaling in preclinical models of alcohol, drug, and food addiction. Int Rev Neurobiol. 2017;136:53–88.
Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–9.
Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–8.
Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30:88–96.
Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–70.
Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46.
Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, et al. (18)F-FECNT: validation as PET dopamine transporter ligand in parkinsonism. Exp Neurol. 2010;226:265–73.
Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, et al. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85.
Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–8.
Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharm Exp Ther. 2004;309:959–69.
Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17:918–25.
Acknowledgements
We would like to thank Michael Rowe, Michelle Bell, Lorne Kerley and Stephanie Rideout for excellent technical assistance and Dr. Leonard Howell for coordinating the [18F]FECNT PET studies. This research was supported by the National Institute on Drug Abuse Grant DA017763.
Author information
Authors and Affiliations
Contributions
MAN designed the experiments. AND, AA-N and SHN performed the behavioral studies, including intravenous catheterization. AND and SHN analyzed the PET data, HDG provided technical guidance with initial PET analyses, KKSS, RJV, AM and MMG were involved in the synthesis of both radiotracers and BAR and MIA were responsible for the statistical analyses. The manuscript was written by MIA and MAN. All authors read a draft of this manuscript and provided critical edits to the content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allen, M.I., Duke, A.N., Nader, S.H. et al. PET imaging of dopamine transporters and D2/D3 receptors in female monkeys: effects of chronic cocaine self-administration. Neuropsychopharmacol. 48, 1436–1445 (2023). https://doi.org/10.1038/s41386-023-01622-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01622-3
This article is cited by
-
Will the promise of translational neuropsychopharmacology research ever deliver? The lion’s roar; the kitten’s purr
NPP—Digital Psychiatry and Neuroscience (2024)
-
Acquisition of cocaine reinforcement using fixed-ratio and concurrent choice schedules in socially housed female and male monkeys
Psychopharmacology (2024)
-
A non-human primate model of cocaine addiction: interpreting associations with increased vs. decreased dopamine function
Neuropsychopharmacology (2023)