Abstract
Hybridization of eggs and sperm from closely related species can give rise to genetic diversity, or can lead to embryo inviability owing to incompatibility. Although central to evolution, the cellular and molecular mechanisms underlying post-zygotic barriers that drive reproductive isolation and speciation remain largely unknown1,2. Species of the African clawed frog Xenopus provide an ideal system to study hybridization and genome evolution. Xenopus laevis is an allotetraploid with 36 chromosomes that arose through interspecific hybridization of diploid progenitors, whereas Xenopus tropicalis is a diploid with 20 chromosomes that diverged from a common ancestor approximately 48 million years ago3. Differences in genome size between the two species are accompanied by organism size differences, and size scaling of the egg and subcellular structures such as nuclei and spindles formed in egg extracts4. Nevertheless, early development transcriptional programs, gene expression patterns, and protein sequences are generally conserved5,6. Whereas the hybrid produced when X. laevis eggs are fertilized by X. tropicalis sperm is viable, the reverse hybrid dies before gastrulation7,8. Here we apply cell biological tools and high-throughput methods to study the mechanisms underlying hybrid inviability. We reveal that two specific X. laevis chromosomes are incompatible with the X. tropicalis cytoplasm and are mis-segregated during mitosis, leading to unbalanced gene expression at the maternal to zygotic transition, followed by cell-autonomous catastrophic embryo death. These results reveal a cellular mechanism underlying hybrid incompatibility that is driven by genome evolution and contributes to the process by which biological populations become distinct species.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seehausen, O. et al. Genomics and the origin of species. Nat. Rev. Genet. 15, 176–192 (2014)
Presgraves, D. C. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 11, 175–180 (2010)
Session, A. M. et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343 (2016)
Brown, K. S. et al. Xenopus tropicalis egg extracts provide insight into scaling of the mitotic spindle. J. Cell Biol. 176, 765–770 (2007)
Hirsch, N., Zimmerman, L. B. & Grainger, R. M. Xenopus, the next generation: X. tropicalis genetics and genomics. Dev. Dyn. 225, 422–433 (2002)
Yanai, I., Peshkin, L., Jorgensen, P. & Kirschner, M. W. Mapping gene expression in two Xenopus species: evolutionary constraints and developmental flexibility. Dev. Cell 20, 483–496 (2011)
Bürki, E. The expression of creatine kinase isozymes in Xenopus tropicalis, Xenopus laevis laevis, and their viable hybrid. Biochem. Genet. 23, 73–88 (1985)
Narbonne, P., Simpson, D. E. & Gurdon, J. B. Deficient induction response in a Xenopus nucleocytoplasmic hybrid. PLoS Biol. 9, e1001197 (2011)
Hamilton, L. Androgenic haploids of a toad, Xenopus laevis. Nature 179, 159 (1957)
Goda, T. et al. Genetic screens for mutations affecting development of Xenopus tropicalis. PLoS Genet. 2, e91 (2006)
Wühr, M. et al. Evidence for an upper limit to mitotic spindle length. Curr. Biol. 18, 1256–1261 (2008)
Cheeseman, I. M. The kinetochore. Cold Spring Harb. Perspect. Biol. 6, a015826 (2014)
Fujiwara, A., Abe, S., Yamaha, E., Yamazaki, F. & Yoshida, M. C. Uniparental chromosome elimination in the early embryogenesis of the inviable salmonid hybrids between masu salmon female and rainbow trout male. Chromosoma 106, 44–52 (1997)
Sakai, C. et al. Chromosome elimination in the interspecific hybrid medaka between Oryzias latipes and O. hubbsi. Chromosome Res. 15, 697–709 (2007)
Ferree, P. M. & Barbash, D. A. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 7, e1000234 (2009)
Kalitsis, P. & Choo, K. H. A. The evolutionary life cycle of the resilient centromere. Chromosoma 121, 327–340 (2012)
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012)
Hatch, E. M., Fischer, A. H., Deerinck, T. J. & Hetzer, M. W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 154, 47–60 (2013)
Terradas, M., Martín, M., Tusell, L. & Genescà, A. DNA lesions sequestered in micronuclei induce a local defective-damage response. DNA Repair (Amst.) 8, 1225–1234 (2009)
Hensey, C. & Gautier, J. A developmental timer that regulates apoptosis at the onset of gastrulation. Mech. Dev. 69, 183–195 (1997)
Vastag, L. et al. Remodeling of the metabolome during early frog development. PLoS ONE 6, e16881 (2011)
Ma, H. et al. Incompatibility between nuclear and mitochondrial genomes contributes to an interspecies reproductive barrier. Cell Metab. 24, 283–294 (2016)
Lee, H. Y. et al. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell 135, 1065–1073 (2008)
Mi, H., Poudel, S., Muruganujan, A., Casagrande, J. T. & Thomas, P. D. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res. 44 (D1), D336–D342 (2016)
Schmid, M. & Steinlein, C. Chromosome banding in Amphibia. XXXII. The genus Xenopus (Anura, Pipidae). Cytogenet. Genome Res. 145, 201–217 (2015)
Nieuwkoop, P. D & Faber, J. Normal Table of Xenopus laevis (Daudin) (Garland, 1994)
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012)
Lee, C., Kieserman, E., Gray, R. S., Park, T. J. & Wallingford, J. Whole-mount fluorescence immunocytochemistry on Xenopus embryos. CSH Protoc. 2008, pdb.prot4957 (2008)
Levy, D. L. & Heald, R. Nuclear size is regulated by importin α and Ntf2 in Xenopus. Cell 143, 288–298 (2010)
Maresca, T. J. & Heald, R. Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts. Methods Mol. Biol. 322, 459–474 (2006)
Murray, A. W. Cell cycle extracts. Methods Cell Biol. 36, 581–605 (1991)
Hannak, E. & Heald, R. Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts. Nat. Protocols 1, 2305–2314 (2006)
Edelstein, A. D. et al. Advanced methods of microscope control using μManager software. J. Biol. Methods 1, 10 (2014)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
Louie, S. M. et al. GSTP1 is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem. Biol. 23, 567–578 (2016)
Acknowledgements
We thank members of the Heald laboratory, present and past, for support and discussions. We thank the students who helped with some of the experiments: B. Castellano, J. Chen, S. Ramos, A. Sabillo, and K. Shih. We are also grateful to the Marine Biological Laboratory and the National Xenopus Resource for organizing the 2013 Advanced Imaging in Xenopus Workshop where several techniques used here were taught to R.G., and to J. Wallingford and A. Shindo for subsequent support. We thank the Welch, King, Harland, Rokhsar, Barton, and Fletcher laboratories at the University of California, Berkeley (UC Berkeley) for sharing reagents, materials, and expertise, as well as T. Stukenberg and A. Straight for providing us with the Ndc80 and CENP-A antibodies, respectively. We especially thank A. Mudd and D. Rokhsar for providing early access to the X. borealis genome assembly. This work used the Functional Genomics Laboratory, a QB3-Berkeley Core Research Facility at UC Berkeley as well as the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by National Institutes of Health (NIH) S10 OD018174 Instrumentation Grant. The confocal microscopy performed in this work was done at the UC Berkeley CRL Molecular Imaging Center, supported by National Science Foundation DBI-1041078. R.G. was initially supported by EMBO long-term fellowship ALTF 836-2013 and for most of this project by Human Frontier Science Program long-term fellowship LT 0004252014-L. R.A. was supported in part by a National Science Foundation REU Summer Fellowship in 2014. R.H. was supported by NIH R35 GM118183 and the Flora Lamson Hewlett Chair. D.K.N. was supported by NIH R01 CA172667. M.K. was supported by UC Berkeley Department of Molecular and Cell Biology NIH training grant 4T32GM007232-40. T.K. was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1C1B2009302), and the UNIST Research Fund (grant number 1.160060.01). G.J.C.V., I.V.K., and G.G. were supported by NIH R01 HD069344.
Author information
Authors and Affiliations
Contributions
R.H. and R.G. designed the project. R.G. performed the molecular, cell, and developmental biology experiments, aided by R.A., and analysed the data. M.K., together with R.G., performed the experiments related to X. borealis and analysed the data. G.J.C.V., I.V.K., and G.G. prepared and analysed the hybrid genomes. B.M. and D.K.N. performed the metabolomic profiling of hybrids. T.K. and E.M.M. contributed to the transcriptome data analysis. R.G. prepared the figures and wrote the manuscript with R.H., incorporating feedback from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks E. Amaya and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Occurrence of micronuclei, role of ploidy and spindle architecture.
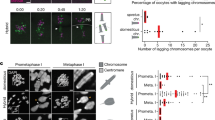
a, Micronuclei in te × ls hybrid embryos at various developmental stages. Whole-mount embryo immunofluorescence was performed in te × ls hybrid embryos using anti-histone H3 antibody at stages 4, 6, 7, 8, and 9 and quantified in b. Scale bar, 10 μm. b, Quantification of micronuclei in te × ls hybrid embryos. The percentage of micronuclei was calculated as the number of micronuclei in the imaged portion of the embryo divided by the total number of nuclei in the same imaged portion. The average percentage for multiple embryos at stage 4 (n = 18 te × ls hybrid embryos (individual dots) with a total of 63 nuclei), stage 6 (n = 17/115), stage 7 (n = 9/322), stage 8 (n = 8/1119), and stage 9 (n = 3/2004) from three independent experiments is shown as thick line. Grey boxes indicate 1 s.e.m. Control X. tropicalis embryos from the same mothers were analysed but no micronuclei were observed at any stages. c, Nuclear size in X. tropicalis embryos with varying ploidy. Nuclear size relative to cell size (diameters in micrometres) is plotted for triploid (tte × ts; dark grey, n = 175 nuclei from six embryos), diploid (X. tropicalis, te × ts; blue, n = 453/9), and haploid (te × [ts]; light grey, n = 346/16) embryos. Each dot indicates an individual data point and the solid lines indicate a linear fit. d, X. tropicalis embryos with varying ploidy at tailbud stage. Images of triploid (tte × ts; left) and haploid (te × [ts]; right) tailbuds were taken under identical conditions. Similar observations were over three independent experiments. e, Size and microtubule distribution in X. tropicalis spindles assembled from different embryo nuclei DNA (n = 147, 103, and 156 spindles quantified for X. tropicalis, le × ts hybrids, and X. laevis embryo nuclei, respectively, from three different egg extracts). Spindle length (left) and width (middle) were normalized to the X. tropicalis control, averages are shown as thick black lines, and the grey boxes indicate 1 s.d. Ninety-five per cent confidence intervals for lengths are 1 ± 0.02 for te × ts, 1.05 ± 0.03 for le × ts, and 1.03 ± 0.02 for le × ls, and for widths are 1 ± 0.04, 1.3 ± 0.07, and 1.4 ± 0.04. Line scans of rhodamine-tubulin signal along spindle length were taken (right). Spindle lengths were normalized to 100% and tubulin intensities were normalized within datasets. The average intensities are plotted for the three spindle types, error bars indicate s.d., and colours are as in Fig. 2a.
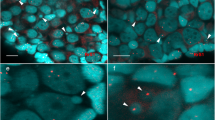
Extended Data Figure 2 Characterization of micronuclei in te × ls hybrid embryos and link to embryo death.
a, Disrupted micronuclei envelopes in te × ls hybrid embryos. Whole-mount embryo immunofluorescence was performed in te × ls hybrid embryos using the YO-PRO DNA dye (top) and anti-Lamin B1 antibody (middle); corresponding channels are shown in green and magenta, respectively. The merged images are shown below. Twenty-five micronuclei within five different embryos were analysed. Intact (left) and disrupted (right) envelopes were observed in all analysed embryos. Scale bar, 10 μm. b, DNA damage in te × ls hybrid embryo micronuclei. Whole-mount embryo immunofluorescence was performed in te × ls hybrid embryos using anti-histone H3 (top) and anti-γH2A.X (middle) antibodies; corresponding channels are shown in green and magenta, respectively. The merge images are shown below. Twenty-one micronuclei within different six embryos were analysed. Micronuclei with undamaged (left; negative γH2A.X signal) and damaged (right; positive γH2A.X signal) DNA were observed in all analysed embryos. Zoomed images of micronuclei are shown on the right of each image. Scale bar, 10 μm. c, TUNEL assay in apoptotic X. tropicalis and te × ls hybrid embryos. X. tropicalis (left), X. tropicalis treated with cycloheximide (middle left) or hydroxyurea (middle right) as indicated, and te × ls hybrid (right) embryos were prepared for TUNEL assay 5 h.p.f. (equivalent stage 9; top), 7 h.p.f. (equivalent stage 10; middle), and 9.5 h.p.f. (equivalent stage 10.5; bottom). Identical results were obtained over three different experiments. Representative images are shown and were taken under identical conditions.
Extended Data Figure 3 Whole-genome sequencing of tte × ls rescued embryos and metabolomic profiling of te × ls and te × bs hybrid embryos.
a, The genomes of 4 tte × ls rescued embryos were sequenced, aligned, and normalized to the genomes of X. tropicalis (blue) and X. laevis (green) for which sub-genomes S and L were distinguished (S in light green and L in dark green). Underrepresented regions of the genomes are colour-coded in black. The tte × ls embryo genomes 1 and 2 were prepared from tailbuds, and 3 and 4 from tadpoles. b, Metabolites differentially represented between te × ls hybrid and X. tropicalis embryos 7h.p.f. Among the 179 metabolites detected, 17 were significantly altered in te × ls hybrid embryos (P < 0.05; two-tailed homoscedastic t-test; individual P values are provided in Fig. 3c source data) and are shown as a ratio to the X. tropicalis control (blue dashed line). Levels were obtained from five samples from three independent fertilizations each. Values for the te × ls hybrid are plotted in orange. The averages are shown as thick lines and the grey boxes correspond to 1 s.d. Ninety-five per cent confidence intervals are, from left to right, 0.69 ± 0.24, 0.46 ± 0.26, 0.16 ± 0.16, 0.68 ± 0.18, 0.70 ± 0.21, 0.58 ± 0.25, 0.10 ± 0.09, 0.42 ± 0.19, 0.38 ± 0.27, 0.79 ± 0.15, 1.61 ± 0.61, 1.47 ± 0.33, 1.58 ± 0.33, 0.83 ± 0.11, 0.71 ± 0.18, 0.70 ± 0.19, and 0.53 ± 0.08. Metabolites with P values below the penalized Bonferroni corrected threshold (n = 12) are labelled in orange. c, Metabolites differentially represented between te × bs hybrid and X. tropicalis embryos 7 h.p.f. Among the 241 metabolites detected, 17 were significantly altered in te × bs hybrid embryos (P < 0.05; two-tailed homoscedastic t-test; individual P values are provided in Fig. 4g source data) and are shown as a ratio to the X. tropicalis control (blue dashed line). Levels were obtained from five samples from three independent fertilizations, each. Values for the te × bs hybrid are plotted in purple. The averages are shown as thick lines and the grey boxes correspond to 1 s.d. Ninety-five per cent confidence intervals are, from left to right, 0.73 ± 0.12, 0.44 ± 0.26, 0.80 ± 0.10, 0.61 ± 0.21, 0.78 ± 0.14, 1.86 ± 0.9, 2.33 ± 1.33, 2.07 ± 1.07, 2.07 ± 1.17, 0.63 ± 0.19, 0.59 ± 0.16 0.61 ± 0.22, 1.39 ± 0.37, 1.51 ± 0.38, 1.24 ± 0.14, 1.21 ± 0.18, and 1.14 ± 0.10. Metabolites with P values below the penalized Bonferroni corrected threshold (n = 3) are labelled in purple.
Extended Data Figure 4 Characterization of micronuclei in te × bs hybrid embryos.
a, DNA damage in te × bs hybrid embryo micronuclei. Whole-mount embryo immunofluorescence was performed in te × bs hybrid embryos using anti-histone H3 (left) and anti-γH2A.X (middle) antibodies; corresponding channels are shown in green and magenta, respectively. The merged image is shown on the right. Thirty-four micronuclei within eight different embryos were analysed. Micronuclei with damaged DNA were observed in all analysed embryos. Zoomed images of micronuclei are shown on the right in the same left-to-right order. Scale bar, 20 μm. b, Micronuclei in te × bs hybrid embryos at various developmental stages (top). Whole-mount embryo immunofluorescence was performed in te × bs hybrid embryos using anti-histone H3 antibody at stages 6, 7, 8, and 9. Scale bar, 20 μm. Quantification of micronuclei in te × bs hybrid embryos (bottom). The percentage of micronuclei was calculated as the number of micronuclei in the imaged portion of the embryo divided by the total number of nuclei in the same imaged portion. The average percentage for multiple embryos at stage 6 (n = 5 te × bs hybrid embryos (individual dots) with a total of 125 nuclei), stage 7 (n = 7/153), stage 8 (n = 9/731), and stage 9 (n = 10/2,691) is shown as a thick line. Grey boxes correspond to 1 s.e.m. Control X. tropicalis embryos from the same mothers were analysed but no micronuclei were observed at any stages. c, Micronuclei size in te × bs and te × ls hybrids. Size is plotted as the ratio between the volumes of the micronucleus and its corresponding nucleus. Each dot represents an individual data point (n = 329 micronuclei from 36 te × bs embryos shown in purple and n = 100 from 17 te × ls embryos shown in orange, from 4 independent experiments). The thick black line indicates the average and the grey box corresponds to 1 s.d. Ninety-five per cent confidence intervals are 2.9 ± 0.36% for te × bs and 1.6 ± 0.28% for te × ls embryos. Statistical significance was shown using a two-tailed heteroscedastic t-test.
Supplementary information
Supplementary Table 1
This table shows chromosomal distribution of lost vs. remaining DNA in te×ls, tte×ls and te×bs hybrids. (XLSX 22 kb)
Supplementary Table 2
This table shows transcriptome profiling of te×ls hybrid compared to X. tropicalis embryos at 7 hpf. (XLSX 677 kb)
Characterization of te×ls hybrid embryo death; X. tropicalis vs. te×ls
X. tropicalis eggs were fertilized with X. tropicalis (left) or X. laevis sperm (right) and simultaneously imaged in separate dishes. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm. (MP4 955 kb)
Cell death in te×ls hybrid animal cap; X. tropicalis vs. te×ls
X. tropicalis eggs were fertilized with X. tropicalis X. tropicalis sperm (left) or X. laevis sperm (right). At stage 8, animal caps were isolated and simultaneously imaged in separate dishes. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm. (MP4 451 kb)
Role of X. laevis DNA in te×ls hybrid embryo death te×ls vs. te×[ls]
X. tropicalis eggs were fertilized with X. laevis sperm (left) or UV-irradiated X. laevis sperm (right) and simultaneously imaged in separate dishes. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm. (MP4 1158 kb)
Development of X. tropicalis haploid embryos; te×[ts] vs. te×[ls]
X. tropicalis eggs were fertilized with UV-irradiated X. tropicalis sperm (left) or UV-irradiated X. laevis sperm (right) and simultaneously imaged in separate dishes. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm. (MP4 704 kb)
Mitosis in te×ls hybrid animal cap
X. tropicalis eggs were fertilized with X. laevis sperm. In vitro transcribed mRNA coding for the microtubule end binding protein EB3 labeled with GFP and histone H2B labeled with RFP was injected into stage 2 embryos. At stage 8, animal caps were isolated, mounted and imaged using live confocal microscopy. Histone H2B-RFP (shown in magenta) and EB3-GFP (shown in green) signals were imaged in a single plane with a frame size of 1024x1024 pixels, every 5s. The movie plays 20 min in 8s (rate of 30 fps). The time is in mm:ss and the scale bar is 20 µm. (MP4 704 kb)
Phenotype of embryo death induced by inhibition of protein synthesis
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged while incubated from stage 6.5 in 1/10X MMR containing 0.1 mg/ml cycloheximide. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Phenotype of embryo death induced by inhibition of DNA replication
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged while incubated from stage 3 in 1/10X MMR containing 30 mM hydroxyurea. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Phenotype of embryo death induced by inhibiting transcription using triptolide
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged in separate dishes while incubated from stage 2 in 1/10X MMR containing 25 μM triptolide (left) or a corresponding amount of DMSO (right). The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Effect of triptolide treatment on te×ls hybrid embryos
X. tropicalis eggs were fertilized with X. laevis sperm and imaged in separate dishes while incubated from stage 2 in 1/10X MMR containing 25 μM triptolide (left) or a corresponding amount of DMSO (right). The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Effect of inhibiting ATP synthase on X. tropicalis embryos
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged in separate dishes while incubated from stage 2 in 1/10X MMR containing 40 μM oligomycin (left) or a corresponding amount of DMSO (right). The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Effect of inhibiting glyceraldehyde-3-P dehydrogenase on X. tropicalis embryos
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged in separate dishes while incubated from stage 2 in 1/10X MMR containing 50 mM iodoacetic acid (left) or a corresponding amount of ddH2O (right). The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Effect of inhibiting glycogen phosphorylase on X. tropicalis embryos.
X. tropicalis eggs were fertilized with X. tropicalis sperm and imaged in separate dishes while incubated from stage 2 in 1/10X MMR containing 270 μM CP-91,149 (left) or a corresponding amount of DMSO (right). The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Characterization of te×bs hybrid embryo inviability; X. tropicalis vs. te×bs.
X. tropicalis eggs were fertilized with X. borealis sperm (left) or X. tropicalis sperm (right) and simultaneously imaged in separate dishes. The video plays 20h in 15s (rate of 120 fps) and the scale bar corresponds to 200 μm.
Rights and permissions
About this article
Cite this article
Gibeaux, R., Acker, R., Kitaoka, M. et al. Paternal chromosome loss and metabolic crisis contribute to hybrid inviability in Xenopus. Nature 553, 337–341 (2018). https://doi.org/10.1038/nature25188
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25188
This article is cited by
-
Deep transcriptome profiling reveals limited conservation of A-to-I RNA editing in Xenopus
BMC Biology (2023)
-
Species delimitation and mitonuclear discordance within a species complex of biting midges
Scientific Reports (2022)
-
The neutral rate of whole-genome duplication varies among yeast species and their hybrids
Nature Communications (2021)
-
Diversification and hybrid incompatibility in auto-pseudogamous species of Mesorhabditis nematodes
BMC Evolutionary Biology (2020)
-
Identifying proteins bound to native mitotic ESC chromosomes reveals chromatin repressors are important for compaction
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.