Abstract
While cross-sectional studies suggest that patients with mood disorders have a higher ratio of omega-6 to omega-3 polyunsaturated fatty acids (PUFAs) and lower levels of omega-3 PUFAs, it is unknown if a high n-6/3 ratio indicates vulnerability for depression. We tested this hypothesis in a 7-year follow-up study of young individuals with an ultra-high risk (UHR) phenotype. We conducted a secondary analysis of the Vienna omega-3 study, a longitudinal study of omega-3 PUFAs in individuals at UHR for psychosis (n=69). Levels of n-6 and n-3 PUFAs were measured in the phosphatidylethanolamine fraction of erythrocyte membranes at intake into the study. Mood disorder diagnosis was ascertained with the Structured Clinical Interview for DSM-IV-TR and confirmed by review of medical records and interviews of caregivers. A higher n-6/3 PUFA ratio at baseline predicted mood disorders in UHR individuals over a 7-year (median) follow-up (odds ratio=1.89, 95% CI=1.075–3.338, P=0.03). This association remained significant after adjustment for age, gender, smoking, severity of depressive symptoms at baseline and n-3 supplementation. Consistent results were obtained for individual PUFAs, including lower levels of eicosapentaenoic acid and docosahexaenoic acid. The predictive capacity of these findings was specific to mood disorders as no associations were found for any other psychiatric disorder. To our knowledge, our data provide the first prospective evidence that the n-6/3 PUFA ratio is associated with an increased risk for mood disorders in young people exhibiting an UHR phenotype. These findings may have important implications for treatment and risk stratification beyond clinical characteristics.
Similar content being viewed by others
Introduction
Long-chain polyunsaturated fatty acids (PUFAs) have important physiological functions and are key regulators of cell membrane properties. As such, they play important structural and functional roles in the human brain and affect monoaminergic neurotransmission, dendritic arborization, synapse formation, and ion channel function.1, 2 Omega-3 (n-3) PUFAs have been shown to possess anti-inflammatory 3 and antioxidant 4 properties, while n-6 PUFAs are generally seen as pro-inflammatory, and a high n-6/3 PUFA ratio is thought to have adverse health effects.5
Epidemiological studies show that the dietary intake of n-3 PUFAs through fish consumption is inversely correlated with the prevalence of depression,6, 7 thus suggesting that n-3 PUFAs have a protective effect. While the traditional human diet has a ratio of n-6/3 PUFA of approximately 1:1, contemporary Western diets are characterized by a ratio of around 15:1, reflecting deficient intake of n-3 fatty acids and excessive intake of n-6 fatty acids.8 Importantly, adherence to a Western diet is associated with a higher prevalence of depression.9 This has given rise to the hypothesis that the long-term changes in dietary patterns that have occurred in industrialized countries over the last centuries have led to an increase in the n-6/3 PUFA ratio,5 which may be causally linked to an increase in the incidence of mood disorders. Indeed, case–control studies have suggested that individuals with depression have lower levels of n-3 PUFAs and higher n-6/3 PUFA ratios compared to healthy controls.10 Recent meta-analyses of n-3 PUFAs supplementation trials in patients with depression have resulted in inconsistent conclusions. Some meta-analyses have concluded that n-3 PUFA supplementation does not have a significant benefit on depressive symptoms,11 while others have found significant beneficial effects,12, 13, 14 which appeared to be related to higher doses of the n-3 PUFA eicosapentaenoic acid (EPA).12, 15, 16 Although lower levels of n-3 PUFAs and higher n-6/3 PUFA ratios have been reported in adults with depression, little is known about PUFA levels in youth, nor whether abnormalities in membrane lipid composition precede the onset of depression in this group. This is salient because even prenatal and early childhood diet appears to play a role in risk for mood disorders, and as such represents a potential intervention target.17
An important mechanistic question is whether individuals with low levels of n-3 PUFAs or an increased n-6/3 PUFA ratio are at elevated risk of developing mental disorders. We have recently demonstrated that higher levels of α-linolenic acid, the ‘parent’ essential fatty acid of the n-3 family, predicted short-term functional improvement among individuals at ultrahigh risk (UHR) of developing psychosis who receive n-3 PUFAs supplementation,18 while decreased levels of nervonic acid, a monounsaturated very long-chain fatty acid involved in myelin synthesis, predicted transition to first-episode psychosis in the same study.19 In contrast, longitudinal studies investigating associations of n-3 PUFAs and depressive symptoms in general adult population samples have not reported significant associations.20, 21
To address the hypothesis that low levels of n-3 PUFAs or an increased n-6/3 PUFA ratio precedes the onset of mood disorders in at-risk groups, we conducted a secondary analysis of the Vienna n-3 study, a 12-week randomized controlled trial (RCT) of n-3 PUFAs or placebo in UHR patients,22 to test whether erythrocyte membrane levels of PUFAs predict mood disorders during a (median) 7-year follow-up in young individuals with UHR for psychosis.
Materials and methods
Study design
The present study is a secondary analysis of a double-blind RCT of n-3 PUFAs for indicated prevention of psychosis transition in individuals at UHR for psychosis (trial registration: clinical trials.gov identifier: NCT00396643). Details of this RCT, including the trial design, interventions, randomization, inclusion and exclusion criteria, blinding and study measures have been published elsewhere.22 Briefly, a 12-week intervention with 1.2 g per day of n-3 PUFAs (700 mg EPA, 480 mg docosahexaenoic acid (DHA)) or placebo was conducted with follow-up at 1 year and 7 years (median) after baseline. The study was approved by the Medical University of Vienna Ethics Committee, and written informed consent was obtained from all participants or participants and their legal guardians for participants aged <18 years.
Participants
Eighty-one treatment-seeking individuals were enrolled in the original study. All participants were consecutive admissions to a specialized psychosis detection and treatment unit at the Department of Child and Adolescent Psychiatry, Medical University Vienna, between 2004 and 2006. Participants were aged 13–25 years at first presentation and met criteria for one or more of the three operationally defined and well-validated risk factors for psychosis proposed by Yung et al.23 attenuated positive psychotic symptoms; transient psychosis; and genetic risk plus a decrease in functioning. The present study sample consists of 69 of the 81 individuals enrolled in the trial (85.2% 47 female, 22 male; mean age 16.38 years, s.d. 1.83) who had information on diagnostic outcomes 7 years (median) after study entry.
Psychometric assessments
The primary outcome measure used for this study was any occurring diagnosis of mood disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria within the 7-year follow-up period, ascertained in a personal interview with the participant using the Structured Clinical Interview for DSM-IV-TR Axis I disorders (SCID-I/P) at the longer term follow-up assessment (median 7 years after enrollment into the study). If a participant was unavailable for an interview, we aimed to establish psychiatric diagnoses using next of kin and/or medical record information. The Global Assessment of Functioning (GAF) scale was used to measure social, occupational, and psychological functioning at baseline and follow-up. The Montgomery–Asberg Depression Rating Scale (MADRS) was used to assess depressive symptoms. Inter-rater reliability estimates were high (intra-class correlation coefficients >0.92). Cronbach’s alpha for the MADRS in this study was 0.89. Inter-rater reliability was maintained by using videotaped interviews every 3 months across the initial 12 months of the RCT and before longer term follow-up.
Analysis of erythrocyte membrane PUFA composition
Erythrocyte membrane phospholipid composition closely reflects that of neuronal membranes and provides an easily accessible indicator of brain phospholipids.24 Erythrocytes were separated from whole blood samples and the PUFA composition of phospholipids of the phosphatidylethanolamine fraction was analyzed from erythrocyte membranes. Phosphatidylethanolamine was chosen because it contains higher levels of PUFAs relative to other phospholipids. We used gas chromatography to determine values for PUFAs and included the following into our analysis: 18:2n-6, 18:3n-6, 20:3n-6, 20:4n-6, 22:2n-6, 22:4n-6, 18:3n-3, 20:5n-3, 22:5n-3, 22:6n-3. The detailed methods and rationale are published in previous reports.25
Statistical analysis
We included all study participants who did not meet the case definition (that is, being diagnosed with a mood disorder within the follow-up period, n=43, Table 1) as the comparison group, which thus consisted of individuals with no psychiatric disorder, but also individuals with psychotic disorders, anxiety disorder and/or other psychiatric disorders.
As an alternative to the conservative Kolmogorov–Smirnov test with Lilliefors correction, normality of data was ascertained by assessing the skewness of data distribution and the associated standard errors. Skewed variables were log transformed, and a range of <3 was used as the cut-off for the use of parametric tests.
Unadjusted between-group differences in demographic variables and fatty acids (FAs) were tested with independent samples t-tests and X2 tests or Fisher’s exact test for continuous and categorical variables, respectively, and group mean values were transformed into effect size measures (Cohen’s d) for comparison of FAs between diagnostic categories. We used repeated measures analysis of variance (ANOVA) to examine changes in scores between and within groups. Logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CIs) for mood disorder diagnosis within the 7-year follow-up period. PUFAs (z-transformed values) and their composite indices were used as independent variables and ORs were calculated per s.d. increase in PUFA levels. All regression models were fitted as unadjusted models, models adjusted for age, gender and smoking and models adjusted for age, gender, smoking, baseline MADRS score and n-3 PUFA/placebo group status. We conducted sensitivity analyses excluding individuals with psychotic disorder outcome from both case and comparison group for all tests. The Hosmer–Lemeshow test was used to test model calibration. All tests were two-sided, a P-value of <0.05 was considered significant. SPSS version 22 was used for all analyses.
Results
Demographics and psychometric measures
The median follow-up duration was 7.14 years (IQR=1.13 years). The sources of information to determine diagnostic outcome were interviews (61 with participants, 4 with next of kin) for 94.2% (65/69) and medical records for 5.8% (4/69) of participants. Of these 69 participants, 26 (37.7%) received a mood disorder diagnosis during the follow-up period (24 major depressive disorder; 2 bipolar II disorder), 20 converted to a psychotic disorder, 19 received an anxiety disorder and 7 a substance use disorder diagnosis. No significant differences in any of the demographic variables were found between participants with and without mood disorder (Table 1). While GAF scores were not significantly different between the two groups at the baseline assessment, we found a significant time by group interaction (F1.000=4.50, P=0.04), indicating that GAF scores changed differentially between individuals with and without a mood disorder diagnosis. Post hoctests revealed that GAF scores did not change significantly during the follow-up period in individuals with mood disorders (baseline 56.9±11.1 vs follow-up 58.1±13.2, P=0.62), whereas individuals with no mood disorders improved significantly (baseline 62.2±13.6 vs follow-up 71.1±16.9, P<0.001). No significant time by group interaction and no between-group differences were found in MADRS scores at baseline and follow-up.
Between-group differences in PUFAs
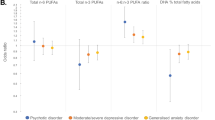
Three n-6 PUFAs, two n-3 PUFAs, the sum of n-3 PUFAs, and the n-6/3 PUFA ratio were differentially elevated or decreased at baseline in patients with subsequent mood disorders relative to the comparison group (Table 2). This effect was specific for mood disorders; no group differences were found for psychotic disorders, anxiety disorders or any other psychiatric disorder. Patients with mood disorders at follow-up had lower baseline levels of linolenic acid (18:2n-6), higher baseline levels of γ -linolenic acid (18:3n-6) and docosadienoic acid (22:2n-6), lower levels of EPA (20:5n-3), lower levels of DHA (22:6n-3), lower sum of n-3 PUFAs, and a higher n-6/3 PUFA ratio. No differences were found between patients with mood disorders at follow-up with previous mood disorder episodes relative to patients with no history of mood disorders (all PUFAs P>0.14). Sensitivity analyses excluding participants who converted to psychosis (n=20) showed that EPA (P=0.009) and the sum of n-3 PUFAs (P=0.04) remained significantly lower in individuals with mood disorder compared to individuals without mood disorder, while linolenic acid (18:2n-6), γ-linolenic acid (18:3n-6), docosadienoic acid (22:2n-6), DHA (22:6n-3), and the n-6/3 PUFA ratio consistently remained at trend level. To exclude the possibility of selection bias, we also tested for differences in PUFA concentrations between participants with and without follow-up data and found that their membrane PUFA profiles did not differ (all PUFAs P>0.277).
Regression analysis of PUFAs for mood disorder
Binary logistic regression analysis with n-6/3 PUFA ratio as the independent variable demonstrated that a higher basal n-6/3 PUFA ratio predicted mood disorder over the 7-year follow-up period (Table 3). The effect remained significant when we adjusted the model for gender, age, baseline MADRS score and n-3/placebo group status. This relationship appeared to be driven by a lower sum of n-3 PUFAs, which was independently predictive of mood disorder diagnosis. A series of logistic regression analyses revealed that linolenic acid (18:2n-6), γ-linolenic acid (18:3n-6), docosadienoic acid (22:2n-6), EPA (20:5n-3), and DHA (22:6n-3) individually predicted mood disorders (Table 3). Sensitivity analyses excluding participants who converted to a psychotic disorder (n=20) showed that EPA (20:5n-3), DHA (22:6n-3) (trend level), and the n-6/3 PUFA ratio (trend level), remained predictive of mood disorders and were thus consistent with our main findings. The significant findings also persisted when the two participants with a Bipolar II diagnosis at follow-up were excluded.
Effect modification by gender
We found a gender difference in docosapentaenoic acid (22:5n-3), such that females had lower levels compared to males (2.09±0.42 vs 2.34±0.39, P=0.01). However, gender did not affect the relationship between any of the PUFAs and mood disorder in the logistic regression models (all P>0.48).
Discussion
In the present study, we tested the hypothesis that n-6/3 PUFA ratio is a risk biomarker26 for mood disorders and found that a higher n-6/3 PUFA ratio in erythrocyte membranes predicts incident mood disorders in young people exhibiting an UHR phenotype. This predictive capacity of n-6/3 PUFA ratio was specific for mood disorders in this cohort, that is, the n-6/3 PUFA ratio did not influence the risk of developing any other psychiatric disorder and persisted after adjusting our model for several confounders, including age, gender, smoking, baseline depressive symptoms, and n-3 supplementation. Cross-sectional studies suggest that people with depression have higher n-6/3 PUFA ratios compared to healthy controls, characterized by overall lower n-3 levels, but normal n-6 levels.10 These observations are in line with the hypothesis that the changes in diet over the past 150 years may have caused an increase in the incidence of depression through increased n-6/3 PUFA ratios. To the best of our knowledge, this study is the first to test the hypothesis that higher n-6/3 PUFA ratios posit a risk for future depression in a longitudinal design with a long (7-year median) follow-up in at-risk individuals.
N-6 PUFAs, n-3 PUFAs, and the balance between the two are important to maintain physiological membrane properties.27 The n-3 PUFA content in the lipid bilayer of mammalian cells determines the physiological functions of the cellular membrane, including membrane fluidity and the function of ion channels and membrane receptors.27, 28 As such, n-3 PUFAs are thought to interact with several pathophysiological mechanisms in depression, including serotonergic neurotransmission 29 and decreased neurogenesis.30 Additionally, depression is characterized by oxidative stress, which preferentially impacts lipids, increasing the turnover of, and hence the demand for, critical lipids, thus aggravating this imbalance.31 Data from rodent studies show that dietary restriction of DHA, for instance, appears to predominantly affect DHA levels in the grey matter of cortical areas, the hippocampus, and the striatum,32, 33 and conversely, supplementation of DHA may have a neuroprotective effect on these brain areas.34
A noteworthy finding is that individuals with a mood disorder during the 7-year follow-up period had significantly lower EPA and DHA levels at baseline, and these two n-3 PUFAs individually predicted mood disorder in our logistic regression models. This finding is in line with evidence from studies showing that EPA and DHA in particular are decreased in patients with depression.10 DHA is highly abundant in the human brain and constitutes up to 40% of the total brain FAs, while EPA accounts only for 1%.35 EPA and DHA are believed to be the main active ingredients in n-3 PUFA supplementation, and importantly, meta-analysis suggests a dose-response relationship between EPA content and clinical efficacy and only supplements with a high EPA content appear to be effective in patients with depression.12, 16
Converging evidence from recent proteomic and transcriptomic studies suggest a modulatory effect of n-3 PUFAs on immune function, including downregulation of transcripts for IL-1beta, IL-6 and TNF-α, and the NFkappaB pathway.36, 37 Consequently, low levels of n-3 PUFAs are thought to be associated with a pro-inflammatory phenotype.3 This view is supported by population-based studies demonstrating that a high n-6/3 PUFA ratio is associated with increased pro-inflammatory cytokines.38 Data from pre-clinical studies show that a high n-6/3 PUFA ratio predisposes mice to an excessive immune reaction when confronted with a lipopolysaccharide immune challenge.39 Moreover, transgenic mice that express n-3 PUFA desaturase, an enzyme that catalyses the conversion from n-6 to n-3 PUFAs that is not naturally expressed in mammalian cells, produce less pro-inflammatory cytokines IL-1β, IL-6 and TNF-α in response to a 24-h lipopolysaccharide challenge, show a higher proportion of more neuroprotective MHC II-positive microglial cells, and are protected from spatial memory deficits.40 One clinical study demonstrated that a high n-6/3 PUFA ratio markedly increased production of IL-6 and TNF-α and patients with depressive symptoms.41 The relevance of these findings is supported by a recent study demonstrating that only patients with depression and high levels of several pro-inflammatory cytokines respond to n-3 PUFA supplementation, while those patients with low inflammation do not respond.42 Collectively, these data suggest that the high n-6/3 PUFA ratio and the low levels of EPA and DHA found in our study may exert their risk for depression partially through immune mechanisms.
Mood disorders were the most common psychiatric diagnosis at follow-up in the Vienna omega-3 study43 and in other samples of individuals at-risk for psychosis,44 affecting up to 42% of individuals who present with an UHR phenotype. Recent research on the frequency of the UHR phenotype in the general population also demonstrated a prevalence of 1.3% and 9.9% for any UHR symptom.45 Furthermore, based on cumulative findings from long-term follow-up studies, the UHR concept has become more explicitly trans-diagnostic or pluripotent.43 This highlights the importance of studying UHR individuals in regard to mood disorder outcomes. Unlike general population samples,20, 21 UHR individuals represent a viable target group for risk stratification using risk biomarkers,26 which adds clinical relevance to our findings. However, the n-3 PUFA intervention raises the question as to whether it had an influence on psychiatric outcomes or if it altered the relationship between membrane PUFAs and psychopathology. We have previously shown that the n-3 PUFA intervention, while effective in preventing psychosis, did not reduce the risk for mood disorders or MADRS scores.43 Patients who received n-3 PUFA and patients who received placebo did not differ in their PUFA profiles at baseline.22 Finally, adjusting our regression models for treatment group did not change the main result. We thus believe that the 12-week intervention does not have implications for the interpretation of the findings of the present study.
Several limitations need to be considered when interpreting the findings of this study. Most importantly, the participants in this study were recruited at a specialized service for early psychosis according to UHR criteria, and thus represent a specific group of young help-seeking individuals. The results of this study might not generalize beyond this setting. Secondly, the relatively small sample size increases the risk of missing smaller effects in some of the variables and warrants replication of our findings. Thirdly, we did not measure dietary intake of n-3 PUFA during the trial and we thus cannot rule out the possibility that the differences in membrane PUFA composition at baseline in participants who later developed a mood disorder were caused by dietary intake. Strengths of the study include the long follow-up period of 7 years, the use of standardized assessment instruments, the robustness of the findings in the sensitivity analysis, and finally the consecutive sample of UHR individuals allowed a comparison group that consisted of individuals with a variety of psychiatric diagnoses and thus showed that our findings are specific for mood disorders. In addition, the finding of reduced EPA and DHA levels in individuals who later developed a mood disorder adds face and biological validity, as both of these PUFAs have been implicated in the pathophysiology of depression. Our findings are clinically relevant since mood disorders are the most frequent psychiatric condition observed in UHR patients. Together with meta-analytic evidence showing the efficacy of supplementation with EPA-predominant formulations in depression, our findings provide a rationale for longer term n-3 supplementation in UHR individuals.
In conclusion, our results highlight the importance of lipid biology for the identification of risk biomarkers among individuals with UHR states by demonstrating a longitudinal association between membrane PUFA levels and mood disorders 7 years later. These findings support the hypothesis that higher levels of n-6 PUFAs and lower levels of n-3 PUFAs, possibly due to long-term changes in dietary intake or differential usage, pose a risk for depression. In the context of young people at risk for mental disorders, these findings may have important implications for risk stratification beyond clinical characteristics.
References
Dyall SC . Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 2015; 7: 52.
Muller CP, Reichel M, Muhle C, Rhein C, Gulbins E, Kornhuber J . Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta 2015; 1851: 1052–1065.
Calder PC . Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851: 469–484.
Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav Immun 2013; 28: 16–24.
Simopoulos AP . Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol 2011; 44: 203–215.
Hibbeln JR . Fish consumption and major depression. Lancet 1998; 351: 1213.
Smith KJ, Sanderson K, McNaughton SA, Gall SL, Dwyer T, Venn AJ . Longitudinal associations between fish consumption and depression in young adults. Am J Epidemiol 2014; 179: 1228–1235.
Simopoulos AP . The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Bioly Med 2008; 233: 674–688.
Jacka FN, Pasco JA, Henry MJ, Kotowicz MA, Nicholson GC, Berk M . Dietary omega-3 fatty acids and depression in a community sample. Nutr Neurosci 2010; 7: 101–106.
Lin PY, Huang SY, Su KP . A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010; 68: 140–147.
Bloch MH, Hannestad J . Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry 2012; 17: 1272–1282.
Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhe HG, Schene AH . Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry 2016; 6: e756.
Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016; 173: 575–587.
Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R . Omega-3 fatty acids for depression in adults. The Cochrane Database of Systematic Reviews 2015; 11: Cd004692.
Martins JG, Bentsen H, Puri BK . Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 2012; 17: 1144–1149, discussion 1163-1147.
Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry 2016; 209: 192–201.
Jacka FN, Ystrom E, Brantsaeter AL, Karevold E, Roth C, Haugen M et al. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: a prospective cohort study. J Am Acad Child Adolesc Psychiatry 2013; 52: 1038–1047.
Amminger GP, Mechelli A, Rice S, Kim SW, Klier CM, McNamara RK et al. Predictors of treatment response in young people at ultra-high risk for psychosis who received long-chain omega-3 fatty acids. Transl Psychiatry 2015; 5: e495.
Amminger GP, Schafer MR, Klier CM, Slavik JM, Holzer I, Holub M et al. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol Psychiatry 2012; 17: 1150–1152.
Astorg P, Bertrais S, Alessandri JM, Guesnet P, Kesse-Guyot E, Linard A et al. Long-chain n-3 fatty acid levels in baseline serum phospholipids do not predict later occurrence of depressive episodes: a nested case-control study within a cohort of middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids 2009; 81: 265–271.
Persons JE, Robinson JG, Ammann EM, Coryell WH, Espeland MA, Harris WS et al. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int J Geriatr Psych 2014; 29: 747–757.
Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010; 67: 146–154.
Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry 1998; 172: 14–20.
Carlson SE, Carver JD, House SG . High fat diets varying in ratios of polyunsaturated to saturated fatty acid and linoleic to linolenic acid: a comparison of rat neural and red cell membrane phospholipids. J Nutr 1986; 116: 718–725.
Smesny S, Milleit B, Hipler UC, Milleit C, Schafer MR, Klier CM et al. Omega-3 fatty acid supplementation changes intracellular phospholipase A2 activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol Psychiatry 2014; 19: 317–324.
Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M . Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol Psychiatry 2015; 20: 152–153.
Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J et al. Comparative effects of DHA and EPA on cell function. Pharmacol Ther 2009; 122: 56–64.
van Meer G, Voelker DR, Feigenson GW . Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9: 112–124.
Schipper P, Kiliaan AJ, Homberg JR . A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav Pharmacol 2011; 22: 324–334.
Ferreira CF, Bernardi JR, Bosa VL, Schuch I, Goldani MZ, Kapczinski F et al. Correlation between n-3 polyunsaturated fatty acids consumption and BDNF peripheral levels in adolescents. Lipids Health Dis 2014; 13: 1–5.
Maes M, Galecki P, Chang YS, Berk M . A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 676–692.
Levant B, Ozias MK, Carlson SE . Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr 2007; 137: 130–134.
Xiao Y, Huang Y, Chen ZY . Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr 2005; 94: 544–550.
Bos DJ, van Montfort SJ, Oranje B, Durston S, Smeets PA . Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: What is the evidence? Eur Neuropsychopharmacol 2016; 26: 546–561.
McNamara RK, Carlson SE . Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 329–349.
Svahn SL, Varemo L, Gabrielsson BG, Peris E, Nookaew I, Grahnemo L et al. Six tissue transcriptomics reveals specific immune suppression in spleen by dietary polyunsaturated fatty acids. PLoS ONE 2016; 11: e0155099.
Rangel-Zuniga OA, Camargo A, Marin C, Pena-Orihuela P, Perez-Martinez P, Delgado-Lista J et al. Proteome from patients with metabolic syndrome is regulated by quantity and quality of dietary lipids. BMC Genomics 2015; 16: 509.
Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006; 91: 439–446.
Hintze KJ, Tawzer J, Ward RE . Concentration and ratio of essential fatty acids influences the inflammatory response in lipopolysaccharide challenged mice. Prostaglandins Leukot Essent Fatty Acids 2016.
Delpech JC, Madore C, Joffre C, Aubert A, Kang JX, Nadjar A et al. Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharmacology 2015; 40: 525–536.
Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R . Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosomc Med 2007; 69: 217–224.
Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 2016; 21: 71–79.
Amminger GP, Schafer MR, Schlogelhofer M, Klier CM, McGorry PD . Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun 2015; 6: 7934.
Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR . Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry 2015; 172: 249–258.
Schimmelmann BG, Michel C, Martz-Irngartinger A, Linder C, Schultze-Lutter F . Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: Findings from the BEAR and BEARS-kid studies. World Psychiatry 2015; 14: 189–197.
Acknowledgements
This work was supported by Grant 03T-315 from the Stanley Medical Research Institute. MEB is supported by a JCU postgraduate research scholarship. SWK is supported by the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HM15C114). CGD is supported by an NHMRC Career Development Fellowship (1061757). GPA was supported by National Health and Medical Research Council Australia (NHMRC) Senior Research Fellowship 1080963. PDM was supported by NHMRC Senior Principal Research Fellowship 1060996. MB is supported by a NHMRC Senior Principal Research Fellowship 1059660. SR is supported by an Early Career Fellowship from the Society for Mental Health Research. The study sponsor had no role in the design or reporting of this study. We thank Dr Jessica Hartmann for assistance and our participants for their commitment to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MB has received grant/research support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, and Woolworths, and has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck Merck, Pfizer, and Servier. The remaining authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Berger, M., Smesny, S., Kim, SW. et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: a 7-year longitudinal study. Transl Psychiatry 7, e1220 (2017). https://doi.org/10.1038/tp.2017.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.190
This article is cited by
-
Highly sensitive hybrid structured based dodecagonal core with double layer hexagonal cladding photonic crystal fiber for food oil health sensing
Journal of Optics (2023)
-
Design of a Terahertz Regime-Based Surface Plasmon Hybrid Photonic Crystal Fiber Edible Oil Biosensor
Plasmonics (2023)
-
RETRACTED ARTICLE: Chemical and Sensory Properties of Olive Oils Obtained by Crossing of ‘Ascolana Tenera’ with ‘Karamürsel Su’, ‘Tavşan Yüreği’ and ‘Uslu’ Varieties
Erwerbs-Obstbau (2023)
-
Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: cross-sectional and longitudinal associations in a general population cohort
Translational Psychiatry (2021)
-
Exploring the therapeutic potential of omega-3 fatty acids in depression
Environmental Science and Pollution Research (2021)