Abstract
The effectiveness of neoadjuvant chemotherapy (NACT) followed by concurrent chemoradiotherapy (CCRT) compared with CCRT alone in nasopharyngeal carcinoma (NPC) patients who presented with cervical nodal necrosis (CNN) is unknown. A total of 792 patients with stage T1-4N1-3M0 NPC and presented with CNN based on magnetic resonance imaging were retrospectively reviewed. Propensity score matching method was used to balance treatment arms for baseline characteristics. Eventually, 508 patients were propensity-matched on a 1:1 basis to create two groups (NACT + CCRT and CCRT groups). Survival rates were calculated by Kaplan–Meier method and differences were compared by using the log-rank test. The 5-year disease specific survival, disease-free survival and distant metastasis-free survival were significantly higher in NACT + CCRT group relative to the matched CCRT group (82.1% vs. 72.5%, P = 0.021; 70.3% vs. 54.1%, P < 0.001; 81.9% vs. 67.3%, P < 0.001, respectively). Although the rates of grade 3–4 leucopenia and mucositis were higher in NACT + CCRT group than CCRT group, compliance with the combined treatment was good and no significant difference was observed between two groups. NACT followed by CCRT was relatively safe and could achieve better survival than CCRT alone in NPC patients with CNN by reducing the risk of death, tumor progression and distant metastasis.
Similar content being viewed by others
Introduction
Due to the particular anatomical location and symptoms of nasopharyngeal carcinoma (NPC), most patients with NPC typically have loco-regionally advanced disease at diagnosis1. Approximately 75–85% of NPC patients have regional lymph node metastases2,3, and up to 44% of these patients reported concurrent cervical nodal necrosis (CNN)4. CNN has previously been reported as a strong, independent negative prognostic factor for overall survival (OS), disease free survival (DFS) and distant metastasis-free survival (DMFS). CNN patients have previously been significantly associated with an approximately 12% reduction in 5-year OS, DFS and DMFS rates relative to non-CNN patients4. Furthermore, approximately 20% of CNN patients subsequently develop metastatic disease4. Given the poor prognosis, it is important to find effective strategies to improve the survival outcomes of this specific subgroup.
Concurrent chemoradiotherapy (CCRT) has been settled as a standard treatment and recommended as a category 2A option for locally advanced NPC5. The five-year OS rate under CCRT could be increased from 59% to 70% when compared to radiation (RT) alone6,7. However, although adding neoadjuvant chemotherapy (NACT) is considered to have the potential to further reduce the risk of distant failure, especially in patients with extensive nodal disease, the value of NACT followed by CCRT remains controversial8,9,10,11. Some studies have reported that NACT may significantly decrease the risk of distant metastasis in locally advanced NPC in addition to CCRT8,12,13, but others failed to show any survival benefit14,15.
Since the value of NACT in NPC patients with CNN has not been clearly demonstrated, we performed this retrospective study to evaluate the role of NACT followed by CCRT in this specific group of patients.
Results
Treatment outcomes
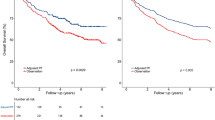
Median follow-up time for the 508 propensity score-matched patients was 50 months (range, 4.5–86 months). At last follow-up, 183 patients (36.0%, 183/508) had experienced treatment failure and 111 (21.9%, 111/508) had died. A total of 74 patients (14.6%, 74/508) developed loco-regional recurrence and 127 patients (25.0%, 127/508) developed distant metastases. The patterns of treatment failure were summarized in Table 1. Fewer patients (n = 71) in NACT + CCRT group experienced treatment failure compared with CCRT group (n = 112), with a lower rate of distant metastases (19.3% vs. 30.7%, P = 0.004). But no difference was found in loco-regional recurrence rate (P = 0.530).
For the entire matched cohort, the 5-year DSS, DFS, RRFS and DMFS rates were 77.4%, 62.3%, 92.1% and 74.7%, respectively. The survival rates of 5-year DSS, DFS and DMFS were significantly higher in NACT + CCRT group relative to the CCRT group (82.1% vs. 72.5%, P = 0.021; 70.3% vs. 54.1%, P < 0.001; 81.9% vs. 67.3%, P < 0.001, respectively), Although no significant difference was observed in RRFS between two groups, there was an improvement tendency in regional control following NACT + CCRT compared with CCRT alone (94.3% vs. 89.6%, P = 0.054)(Fig. 1).
Subgroup analysis
Subgroup analyses demonstrated that patients with different NACT regimen combinations (TPF, PF or TP) were associated with similar survival rates (Supplementary Figure 1). No difference in DSS, DFS, DMFS was observed between different NACT cycles (≤2 cycles vs. >2 cycles), with 5-year DSS, DFS and DMFS rates of 81.3% vs. 85.5% (P = 0.698), 71.0% vs. 68.0% (P = 0.698), 81.8% vs. 82.7% (P = 0.824), respectively. However, the 5-year RRFS was significantly higher in patients ≤2 cycles than those >2 cycles (96.1% vs. 87.3%, P = 0.017). (Supplementary Figure 2)
Univariate and Multivariate analysis
Univariate analysis revealed age (>44 years), gender (male), T stage, clinical stage, treatment of NACT + CCRT were prognostic factors (Supplementary Table). Multivariate analyses demonstrated that the use of NACT + CCRT could significantly reduce the risk of death, tumor progression and distant metastasis compared with CCRT alone. The risk of distant metastasis could be reduced over 50% (HR = 0.49; 95% CI, 0.34–0.71) by addition of NACT to CCRT. T stage was an independent prognostic factor for DFS and DMFS (P = 0.013 and P = 0.003, respectively). Gender (male) was an independent negative prognostic factor for DSS, DFS and DMFS (P = 0.01, 0.028 and 0.008, respectively). Age >44years was an independent negative prognostic factor for DSS (P = 0.037) (Table 2).
Acute toxicities
No grade 5 acute toxicity events (i.e. death) were observed in the matched sample during treatment. The rate of severe acute toxicities (grade 3–4) was higher in NACT + CCRT group relative to the CCRT group, however this difference was not statistically significant. A total of 110 patients (43.3%, 110/254) in the NACT + CCRT group and 91 (35.8%, 91/254) in the CCRT group experienced grade 3–4 acute treatment related adverse events (P = 0.102). The most common grade 3–4 hematological toxicity was leucopenia in 19.7% patients (n = 50) in the NACT + CCRT group and 13.4% patients (n = 34) in the CCRT group (P = 0.073). Grade 3–4 thrombocytopenia was found in 13 patients (5.1%, 13/254) and 7 patients (2.8%,7/254) in the NACT + CCRT group and CCRT group, respectively (P = 0.254). The most frequently recorded grade 3–4 non-hematological acute treatment related adverse events were mucositis, with 28.3% patients (n = 72) in the NACT + CCRT group and 20.9% patients (n = 53) in the CCRT group (P = 0.064). (Table 3)
Discussion
To the best of our knowledge, this is the first study to explore the use of NACT followed by CCRT in the treatment of NPC patients diagnosed with CNN. We demonstrated that NACT followed by CCRT could improve DSS, DFS and DMFS in this particular group of patients, when compared with CCRT alone. After adjustment with other clinical factors in multivariate analysis, NACT + CCRT remained associated with a significant reduction in the rate of death, progress and distant metastasis. These results might give us some directions to select proper patients who might benefit most from the combination of NACT and CCRT, and help guide us to use NACT more effectively.
With the extensive adoption of CCRT as well as the emergence of highly precise RT techniques such as IMRT, the local-regional control rate in NPC has been considerably improved, with distant metastasis taking over as the predominant mode of treatment failure16,17,18,19. Therefore, NACT gradually came to our attention, due to its potential of reduction in both local and distant failures, although no significant improvement in overall survival attributable to NACT has previously been observed20,21. Our study demonstrated that NACT could significantly improve the survival of the specific group of patients with CNN. It was consistent with several studies which showed that NACT could significantly reduce the hazard of progression and distant failure in locally advanced NPC followed by CCRT8,12,13,22. Song et al12. reported that NACT + CCRT performed significant treatment effect in progressive free survival (HR = 0.66, 95% CI 0.49–0.90) and distant metastasis failure-free survival (HR = 0.60, 95% CI 0.39–0.98), when comparing with CCRT alone.
However, other studies failed to show any survival benefit from adding NACT to CCRT14,15. Because of the discordance of results received in different retrospective and clinical trials, the role of adding NACT to CCRT remains controversial8,9,10,11. The 2015 NCCN Guidelines® only recommends it as a category 3 option for patients with NPC with T1,N1-3 or T2-4, any N lesions5. One possible explanation for the failure of NACT to improve survival in previous studies was that NACT might only be of benefit in certain high-risk group of patients with high potential for metastasis, but not all general advanced stage III-IV candidates enrolled in clinical trials. The predominant advantage of NACT was that it might be helpful eradicating the distant micro-metastases existing before radiation, not the new distant metastases emerged after treatment23. So the patients who might benefit most from NACT would be those who most likely had distant micro-metastases before radiation, such as patients with CNN in our study, but not all general stage III-IV patients9. The incidence of CNN in NPC patients with positive regional node metastases was as high as 44%. For this large number of patients who experienced poor DMFS4, appropriate treatment should be warranted.
Subgroup analyses showed that cisplatin-based NACT were generally tolerable, and no difference in survival was observed between different regimens, which were consistent with previous studies24,25. Over 90% of patients (n = 229) received two or more cycles of NACT, and no significant difference in the treatment efficacy between different cycles of chemotherapy was reported, with the exception of an improvement in RRFS using NACT ≤2 cycles. However, the small number of patients receiving >2 cycles of NACT (n = 51) suggests that these findings would require confirmation with larger studies.
Similar to other studies, acute toxicities were acceptable8,9. Although the rates of severe acute adverse events were higher in NACT + CCRT group, particularly with regards to leucopenia and mucositis, most of these toxicities were reversible.
A limitation of this study is the retrospective nature and single institute design. Although the propensity score match was used to eliminate personal bias between two groups, the results from larger, prospective randomized trials are required. Moreover, with the relatively short follow-up time (median, 50 months) and lack of complete late toxicity data, a long-term investigation was necessary.
In conclusion, NACT followed by CCRT could achieve better survival compared with CCRT alone in NPC patients diagnosed with CNN by reducing the risk of death, tumor progression and distant metastasis. Randomized trials are needed to further validate the value of NACT in this specific group of patients.
Methods
Patients
A total of 792 consecutive patients in previous study with newly histological-proven T1-4N1-3M0 NPC diagnosed with CNN based on MRI between January 2007 and December 2009 were enrolled in this study4. All patients were treated by definitive RT ± chemotherapy according to our institutional protocol. Restaging was performed according to the American Joint Committee on Cancer (AJCC) 2010 staging system26. The study was approved by the Research Ethics Committee of Sun Yat-sen University Cancer Center. All the methods were carried out in accordance with approved guidelines of our institute. Written informed consent was obtained from all patients prior to therapy.
Inclusion criteria included: (1) consecutive patients between January 2007 and December 2009 with a pathological diagnosis of nasopharyngeal squamous cell carcinoma; (2) complete MR images of the nasopharynx and neck; (3) all patients were diagnosed with cervical nodal necrosis based on MRI; (4)all patients were treated with concurrent chemoradiotherapy. Exclusion criteria included: 1) patients without complete MR images of the nasopharynx and neck; (2) patients without cervical nodal metastasis; (3) patients with at least one distant metastasis; (4) patients who did not have concurrent chemotherapy or had not completed radiation therapy. A total of 209 patients who did not receive CCRT were excluded from our study (147 patients received NACT + RT, 62 patients received RT alone). Of the 583 patients treated with CCRT, 262 were treated with NACT followed by CCRT while the remaining 321 patients received CCRT alone. The baseline characteristics of the patients were not balanced in two groups prior to matching. Patients in the NACT + CCRT group had more advanced T stage (T4), N stage (N3) and clinical stage (stage IV) disease relative to the unmatched CCRT arm (all P < 0.05). Demographic and clinical variables used to derive the propensity score model included age, gender, histological type, T stage, N stage, clinical stage and RT technique. Eventually, 508 patients were matched on the propensity score to create two groups each containing 254 patients. The characteristics of the patients were well-balanced between the propensity-matched groups (Table 4).
Work-up
Pretreatment evaluation consisted of a complete physical examination, hematologic and biochemistry profiles, MRI scans of the nasopharynx and neck, chest radiography or computed tomography (CT), abdominal sonography, and emission computed tomography (ECT) or whole-body positron emission tomography/CT. Nasopharynx and cervical MR images were available for all patients and were used to assess lymph nodes status. The imaging protocol and the criteria for diagnosis of CNN have been reported in detail in previous study4. The criteria for diagnosis of CNN on MRI were a focal area of high signal intensity on T2WI images or a focal area of low signal intensity on T1WI + C images with or without a surrounding rim of enhancement. (Fig. 2).
Treatment
All patients were treated with definitive RT covering the nasopharynx and retropharyngeal lymph nodes within the primary target. Whole-neck irradiation was performed in all cases. Over half of the patients (264/508; 52.0%) were treated by conventional techniques, 45.7% (232/508) by intensity modulated radiation therapy (IMRT), and 2.3% (12/508) by three-dimensional conformal radiation therapy (3D-CRT). The details of the RT techniques used at our institute have been reported previously16,27.
The NACT regimens included PF (75–80 mg/m2 cisplatin on day 1 and 800 mg/m2/d fluorouracil civ on days 1–5), TPF (70–75 mg/m2 docetaxel on day 1, 70–75 mg/m2 cisplatin on day 1 and 650–700 mg/m2/d fluorouracil civ on days 1–5) or TP (75 mg/m2 docetaxel on day 1 and 75 mg/m2 cisplatin on day 1); the regimens were repeated every 3 weeks. PF, TPF, TP were administered in 132, 53 and 69 patients, respectively. Two-hundred and three patients received 2 cycles NACT and only 51 patients received over 3 cycles. The CCRT regimen was 80–100 mg/m2 cisplatin repeated every 3 weeks or 40 mg/m2 weekly. Patients receiving other chemotherapy regimens were excluded from this study.
Follow-up
Follow-up was calculated from the day of diagnosis to the date of the event or last follow-up visit. All patients were followed-up every 3 months for the first 3 years, every 6 months for the next 2 years, and then annually. Toxicity was assessed according to the Radiation Therapy Oncology Group criteria28 or the Common Terminology Criteria for Adverse Events [CTCAE] v3.029. Disease-specific survival (DSS) was calculated from the day of diagnosis to death caused by cancer at the date of last follow-up; disease–free survival (DFS) was calculated from the day of diagnosis to loco-regional relapse, distant relapse or tumor-related death; regional recurrence–free survival (RRFS) was calculated from the day of diagnosis to regional relapse; distant metastasis–free survival (DMFS) was calculated from the day of diagnosis to first observation of distant lesion(s).
Statistical analysis
A propensity score matching method30 was performed to match the patients from the NACT + CCRT group to comparable patients in the CCRT group on a 1:1 basis. Propensity scores were calculated by logistic regression for patients based on the listing covariates: age, gender, histological type, T stage, N stage, clinical stage and RT techniques. Covariates balance between groups was examined by chi–square test or fisher exact test (categorical variable) as appropriate. Survival rates were calculated using the Kaplan-Meier method and differences were compared by using the log-rank test. A multivariate Cox proportional hazards model was used to calculate hazard ratios (HR), 95% confidence intervals (CI) and test the independent significance of different factors by backward elimination of insignificant factors. A two-tailed P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS v 22.0 (Chicago, IL, USA) and STATA version14.0.
Additional Information
How to cite this article: Lan, M. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in nasopharyngeal carcinoma patients with cervical nodal necrosis. Sci. Rep. 7, 42624; doi: 10.1038/srep42624 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ma, B. B. & Chan, A. T. Systemic treatment strategies and therapeutic monitoring for advanced nasopharyngeal carcinoma. Expert review of anticancer therapy 6, 383–394 (2006).
Ho, F. C., Tham, I. W., Earnest, A., Lee, K. M. & Lu, J. J. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC cancer 12, 98 (2012).
Loong, H. H., Ma, B. B. & Chan, A. T. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematology/oncology clinics of North America 22, 1267–1278 (2008).
Lan, M. et al. Prognostic Value of Cervical Nodal Necrosis in Nasopharyngeal Carcinoma: Analysis of 1800 Patients with Positive Cervical Nodal Metastasis at MR Imaging. Radiology 276, 536–544 (2015).
Pfister, D. G., S. S., Brizel, D. M. et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers Version 1.2015. ©2015 National Comprehensive Cancer Network, Inc. Available at: www.NCCN.org [accessed 02.02.15].
Chan, A. T. et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Journal of the National Cancer Institute 97, 536–539 (2005).
Sze, H., Blanchard, P., Ng, W. T., Pignon, J. P. & Lee, A. W. Chemotherapy for Nasopharyngeal Carcinoma–Current Recommendation and Controversies. Hematology/oncology clinics of North America 29, 1107–1122 (2015).
Hui, E. P. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27, 242–249 (2009).
Fountzilas, G. et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 23, 427–435 (2012).
Bae, W. K. et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol 65, 589–595 (2010).
Langendijk, J. A., Leemans, C. R., Buter, J., Berkhof, J. & Slotman, B. J. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 22, 4604–4612 (2004).
Song, Y., Wang, W., Tao, G. & Zhou, X. Survival benefit of induction chemotherapy in treatment for locally advanced nasopharyngeal carcinoma–A time-to-event meta-analysis. Oral oncology 51, 764–769 (2015).
Chen, Y. P. et al. Efficacy of the Additional Neoadjuvant Chemotherapy to Concurrent Chemoradiotherapy for Patients with Locoregionally Advanced Nasopharyngeal Carcinoma: a Bayesian Network Meta-analysis of Randomized Controlled Trials. Journal of Cancer 6, 883–892 (2015).
Song, J. H. et al. The Role of Neoadjuvant Chemotherapy in the Treatment of Nasopharyngeal Carcinoma: A Multi-Institutional Retrospective Study Using Propensity Score Matching Analysis. Cancer research and treatment: official journal of Korean Cancer Association, doi: 10.4143/crt.2015.265 (2015).
Tan, T. et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics 91, 952–960 (2015).
Sun, X. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 110, 398–403 (2014).
Kam, M. K. et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. International journal of radiation oncology, biology, physics 60, 1440–1450 (2004).
Ou, X. et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget 6, 38381–38397 (2015).
Wee, C. W. et al. Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy. Radiation oncology journal 33, 98–108 (2015).
International Nasopharynx Cancer Study Group Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(>or=N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. International journal of radiation oncology, biology, physics 35, 463–469 (1996).
Chua, D. T. et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23, 1118–1124 (2005).
Ma, J. et al. OP0010 Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: Preliminary results of a phase 3 multicentre randomised controlled trial. European Journal of Cancer 50, e3–e4 (2014).
Al-Amro, A. et al. Neoadjuvant chemotherapy followed by concurrent chemo-radiation therapy in locally advanced nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics 62, 508–513 (2005).
Casanova, M. et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol 77, 289–298 (2016).
Li, W. F. et al. Propensity-matched analysis of three different chemotherapy sequences in patients with locoregionally advanced nasopharyngeal carcinoma treated using intensity-modulated radiotherapy. BMC cancer 15, 810 (2015).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17, 1471–1474 (2010).
Luo, W., Deng, X. W. & Lu, T. X. Dosimetric evaluation for three dimensional conformal, conventional, and traditional radiotherapy plans for patients with early nasopharyngeal carcinoma. Ai zheng=Aizheng=Chinese journal of cancer 23, 605–608 (2004).
Cox, J. D., Stetz, J. & Pajak, T. F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). International journal of radiation oncology, biology, physics 31, 1341–1346 (1995).
Trotti, A. et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13, 176–181 (2003).
Austin, P. C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Statistics in medicine 27, 2037–2049, doi: 10.1002/sim.3150 (2008).
Acknowledgements
We would like to thank all the patients who participated in this study and their families, the medical, nursing, and research staffs at San Yat-Sen University Cancer center. We thank Professor Qing Liu from the Department of Cancer Prevention Research in San Yat-Sen University Cancer center for providing us with suggestions on statistical analysis. This research was supported by Grants from the Natural Science Foundation of China (81402244).
Author information
Authors and Affiliations
Contributions
L.M., C.C.Y., H.Y., T.X.L. and L.J.F contributed to the study concepts; L.M., C.C.Y., H.Y., H.F., T.L. and W.S.X., contributed to the study design and data acquisition; L.M., C.C.Y., H.Y., H.F., D.Z.J., L.J.F., S.T.T., P.A., D.M.L., Z.L., T.L., W.S.X. and T.X.L. did the data analysis, interpretation and manuscript editing as well as review.
Corresponding authors
Ethics declarations
Competing interests
All authors are in agreement with the content of the manuscript and no authors have any potential conflicts of interest to disclose. All authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lan, M., Chen, C., Huang, Y. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in nasopharyngeal carcinoma patients with cervical nodal necrosis. Sci Rep 7, 42624 (2017). https://doi.org/10.1038/srep42624
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42624
This article is cited by
-
Prognostic value of cervical nodal necrosis on staging imaging of nasopharyngeal carcinoma in era of intensity-modulated radiotherapy: a systematic review and meta-analysis
Cancer Imaging (2022)
-
Strand-specific miR-28-3p and miR-28-5p have differential effects on nasopharyngeal cancer cells proliferation, apoptosis, migration and invasion
Cancer Cell International (2019)
-
Neoadjuvant chemotherapy followed by radical hysterectomy for stage IB2-to-IIB cervical cancer: a retrospective cohort study
International Journal of Clinical Oncology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.