Abstract
Human bladder smooth muscle cells (HBSMCs) were subjected to pressure cycles of up to 200 cm H2O to a pressure of 0 cm H2O for 24 hours. The total RNA extracted from each group was subjected to microarray analysis. miR-3180-5p emerged as the most overexpressed of all the differentially expressed microRNAs, and this finding was validated by PCR. We then used CCK-8 to quantify cell proliferation after liposome-mediated transfection. Subsequently, we investigated the change in PODN and its downstream signaling proteins, including cyclin-dependent kinase 2 (cdk2) and p21. In addition, flow cytometry was performed to quantify cell-cycle distribution. The results show that miR-3180-5p, the microRNA that was most overexpressed in response to HP, reduced the expression of PODN and podocan (p = 0.004 and p = 0.041, respectively). Silencing of PODN via miR-3180-5p overexpression revealed a significant promotion of cell proliferation increased in the CCK-8 experiment, p = 0.00077). This cell proliferation was accompanied by an increase in cdk2 expression (p = 0.00193) and a decrease in p21 expression (p = 0.0095). The percentage of cells in (S + G2/M) improved after transfection (p = 0.002). It was apparent that HP upregulates miR-3180-5p, which inhibits the expression of PODN and promotes HBSMC proliferation via the cdk2 signaling pathway.
Similar content being viewed by others
Introduction
Mechanical stimuli are the key regulators of cell structure and function in both normal and diseased conditions1. A healthy bladder is exposed to cyclic variations in hydrostatic pressure during the normal course of urine filling and emptying2. Bladder smooth muscle cells (BSMCs), a major constituent of the bladder wall, represent the main cell type that is exposed to hydrostatic pressure (HP). Furthermore, bladder voiding cycles are regulated by human BSMCs (HBSMCs), which are the predominant group of cells that sense mechanical stimuli (viz., hydrostatic pressure on the bladder) and that translate the stimuli into molecular signals by altering the expression of proteins such as integrins, mitogen-activated protein kinases 1/2, and PI3K/SGK1 in order to alter the contractile force of the entire bladder3,4,5. Although the molecules involved in such regulation of bladder responses are known, the detailed mechanisms that translate mechanical stimuli into contractile responses remain unclear.
MicroRNAs are evolutionarily conserved, small noncoding RNAs that regulate approximately 30% of protein-coding gene expression at the post-transcriptional level. Hence, they regulate a wide variety of biological processes, including cell differentiation, growth, and apoptosis6,7,8,9. Increasing evidence suggests that microRNAs are involved in the proliferation of smooth muscle cells (SMCs) (e.g., vascular and airway SMCs)10,11. However, the role of microRNAs in the regulation of HBSMC proliferation in conditions other than bladder carcinomas remains poorly understood12. Furthermore, the induction of specific microRNAs in response to hydrostatic pressure remains unknown.
In the present study, we investigated whether hydrostatic pressure can induce microRNA expression and whether differentially expressed microRNAs are involved in the regulation of BSMC proliferation. Furthermore, we attempted to examine the molecular mechanisms by which such microRNAs may exert their regulatory effect.
Results
Culture and identification of HBSMCs
Before the experiment, we used immunofluorescence to measure the condition of the cells. More than 95% of the cells showed positive immunofluorescent staining for the SM cell marker α-SM actin (Fig. 1).
Altered microRNA expression in response to hydrostatic pressure
The total RNA isolated from HBSMCs 24 h after the application of hydrostatic pressure was used for microarray and data analyses, which revealed the differential expression of specific microRNAs between the cells exposed to hydrostatic pressure and control cells. The HBSMCs contained 103 upregulated microRNAs and 60 downregulated microRNAs in response to hydrostatic pressure (Fig. 2). After target forecasting and functional analysis of these differentially regulated microRNAs, nine upregulated and four downregulated microRNAs were found to be related to cell proliferation. The upregulated microRNAs included miR-3180-5p, miR-3189-3p, miR-369-5p, miR-4283, miR-4287, miR-4323, miR-483-3p, miR-501-5p, and miR-552-3p (Table 1), and the downregulated microRNAs included miR-106b-5p, miR-17-3p, miR-374c-5p, and miR-543 (Table 1).
Hierarchical clustering and stem analysis of the microRNAs.
The heat map diagram shows the result of the two-way hierarchical clustering of miRNAs and samples. Each row represents an miRNA, and each column represents a sample. The miRNA-clustering tree is shown on the left, and the sample-clustering tree appears at the top. The color scale shown at the top illustrates the relative expression level of an miRNA in the certain slide: red represents high relative expression levels, and green represents low relative expression levels. To analyzethe different expressions of microRNAs according tothe stem analysis, all of the altered microRNAs have continually changing trends in the stem analysis.
Validation of microarray results by RT-PCR
Microarray results were validated by RT-PCR. MiR-3180-5p and miR-4323 were significantly upregulated (p = 0.043 and p = 0.027, respectively; Fig. 3a), whereas miR-543 was significantly downregulated (p = 0.048; Fig. 3b). In addition, miR-3180-5p was the microRNA most upregulated in response to hydrostatic pressure (Fig. 3a); thus, it was selected for further validation experiments. This finding indicated that miR-3180-5p was likely involved in the process of HBSMC proliferation.
Different microRNA expressions were validated by PCR in the HBSMCs between the hydrostatic pressure (HP) and the control static group.
(a) Of all the microRNAs, only miR 3180-5p and miR 4323 were significantly increased (p = 0.043, p = 0.027); (b) only miR 543 was significantly decreased (p = 0.048).
Hydrostatic pressure-induced miR-3180-5p participates in proliferation
We focused on miR-3180-5p because it was the most upregulated by hydrostatic pressure. Hydrostatic pressure is known to increase HBSMC proliferation3,4,5. Hence, we tested cell proliferation via a CCK-8 experiment after transfecting HBSMCs with either miR-3180-5p mimics or a miR-3180-5p inhibitor, which increased and decreased the intracellular levels of microRNA, respectively. The cell viability was markedly increased in the mimics group (p = 0.00077) and markedly attenuated in the inhibitor group (p < 0.00001). Thus, miR-3180-5p regulates HBSMC proliferation under hydrostatic pressure. All of these results are presented in Fig. 4.
PODN expression was suppressed by miR-3180-5p
Previous research revealed that miR-3180-5p is involved in HBSMC proliferation. The expression levels of ADARB1 and PODN in the HBSMCs were validated by RT-PCR and western blot to investigate the effect of the overexpression of miR-3180-5p on target mRNA. In cells transfected with miR-3180-5p mimics, the mRNA level of PODN was found to be decreased (p = 0.004), whereas that of ADARB1 was found to be increased (p = 0.008). We corroborated this result at the protein level via western blot. Our study revealed that the expression of podocan, the functional protein product of the PODN gene, could be suppressed by miR-3180-5p overexpression in HBSMCs (p = 0.041). However, adenosine deaminase RNA-specific B1, the functional protein of ADARB1, showed no changes (p = 0.134) in response to miR-3180-5p. Moreover, the levels of PODN and podocan were demonstrably increased in cells transfected with the miR-3180-5p inhibitor (p = 0.0035; p = 0.025). All of these results are shown in Fig. 5(a–f).
The miRNA 3180-5p regulates the expression of PODN.
After upregulation of miR 3180-5p by transfection, (a) the mRNA level of ADARB1was increased (p = 0.008), and (b) the mRNA level of PODN was markedly decreased (p = 0.001). (c) Adenosine deaminase RNA-specific B1, the functional protein of ADARB1, had no change (p = 0.134). (d) The protein level of PODN was markedly decreased in the miR 3180-5p mimics-treated cells. After downregulation of miR 3180-5p via transfection, (e) the mRNA level of PODN was markedly increased (p = 0.0035), and (f) the protein level of PODN was markedly increased (p = 0.025). (g,h) T293T cells were co-transfected by psiCHECK-PODN (wild or mutant type) with mimics miR. The results showthat upon transfection with wild-type psiCHECK-PODN reporter vectors, luciferase activity was decreased by ~41% (p = 0.0022); moreover, mutation of the binding site markedly suppressed the effect of miR 3180-5p (p = 0.8127).
PODN was directly inhibited by miR-3180-5p
Computational prediction of human microRNA targets using miRanda and TargetScan indicated that PODN is a potential target of miR-3180-5p. To establish the direct effect of mi- 3180-5p on the 3′ UTR of PODN, a dual luciferase assay was performed. Luciferase activity was decreased by ~41% (p = 0.0022) when wild-type psiCHECK-PODN reporter vectors were co-transfected with miR-3180-5p in 293T cells. In contrast, the luciferase activity of 293T cells transfected with mutant psiCHECK-PODN showed no significant difference from the NC group (p = 0.8127; Fig. 5g,h).
Expression of cdk2 is promoted by miR-3180-5p
To further validate the inhibitory effect of miR-3180-5p on podocan expression, we investigated the effect of miR-3180-5p on the proteins p21 and cdk2, which function downstream of podocan. Figure 6 shows that decreased expression of podocan was associated with an increase in cdk2 expression (Fig. 6a, p = 0.002) and a decrease in p21 expression (Fig. 6b, p = 0.0095), while increased expression of podocan was associated with a decrease in cdk2 expression (Fig. 6c, p = 0.0002) and an increase in p21 expression (Fig. 6d, p = 0.011). The above results suggest that the function of cdk2 was affected by miR-3180-5p via the regulation of podocan.
The expression of downstream proteins (cdk 2 signaling pathway).
(a) The decrease in the expression of podocan was associated with an increase in cdk2 expression (p = 0.002) (b) and a decrease in p21 expression (p = 0.0095). (c) The increase in expression of podocan was associated with a decrease in cdk2 expression (p = 0.0002) (d) and increase in p21 expression (p = 0.011).
miR-3180-50 facilitated the G1 to S transition in the cell cycle
After confirming the regulation of cdk2 by miR-3180-5p, flow cytometry was performed to test the effect of miR-3180-5p on the cell cycle by calculating the change in the percentage of cells in (S + G2/M). We transfected HBSMCs with miR-3180-5p mimics, and the increased intracellular levels of miR-3180-5p were accompanied by a significant increase (p = 0.002) in the proliferation index (the percentage of cells in (S + G2/M)) of HBSMCs, from 29.32 ± 6.25% in the NC group to 50.50 ± 3.34% in the cells transfected with miR-3180-5p (Fig. 7a,b). This result demonstrated that overexpression of miR-3180-5p, which is involved in the cell cycle, induced cells to progress from G1 to S phase, thus promoting the proliferation of HBSMCs. This result is consistent with our previous study, which showed that application of hydrostatic pressure resulted in an increase in the cell proliferation index from 25.8 ± 7.1% under static conditions to 39.7 ± 4.2% under HP3,4.
The effect of miRNA 3180-5p on the cell cycle.
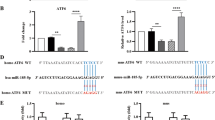
The expression of miRNA 3180-5p and PODN in patient samples. (a,b) The proliferation index, which is the percent of (S + G2/M), increased from 29.32 ± 6.25 in the NC group (non-targeting control sequence-treated cells) to 50.50 ± 3.41 in the miR 3180-5p mimics group (p = 0.002). The miR 3180-5p promoted cell transformation from the G1 to S phase. After induction of hydrostatic pressure, the expression of miRNA 3180-5p was up-regulated ((c,d) p < 0.00001), while the expression of PODN was down-regulated ((e,f) p < 0.00001).
The expression of miR-3180-5p and PODN in patient cells
Next, the expression levels of miR-3180-5p and PODN were tested in patient cells. In samples from patient cells, expression of miR-3180-5p was upregulated under hydrostatic pressure (Fig. 7c,d; p < 0.00001), whereas that of PODN was downregulated (Fig. 7e,f; p < 0.00001). These results were consistent with a previous study, confirmed the clinical relevance of miR-3180-5p, and demonstrated that a better understanding of the relationship between miR-3180-5p and proliferative signaling pathways may provide important novel targets for engineering bladder tissue.
Discussion
Increasing evidence has demonstrated that mechanical stimulation plays an important role in the biological function of the urinary system1,3,4,5. MicroRNAs have emerged as potent and plausible regulators of biological responses6,7,8,9. Furthermore, HBSMCs exposed to mechanical stimulation are known to proliferate, but the molecular mechanisms involved in this process are not clearly understood10,13,14. Hence, we investigated the potential role of microRNAs in the regulation of HBSMC proliferation. Overall, our results demonstrated that high hydrostatic pressure leads to an increase in the expression of miR-3180-5p, which promotes HBSMC proliferation by the suppression of PODN, thereby leading to the activation of the pro-proliferative cdk2 pathway. To our knowledge, the identification of miR-3180-5p as a mechano-sensitive microRNA in HBSMCs represents the first evidence linking microRNA to cell proliferation in the bladder, and miR-3180-5p may be an important target for treatment of bladder diseases.
In this study, we focused on the mechanical stimulation of HBSMCs, as would be the case in vivo during routine bladder filling and voiding cycles, and we observed the differential expression of a distinct group of microRNAs. Using a genome-wide microarray, we identified nine upregulated microRNAs and four downregulated microRNAs in HBSMCs in response to hydrostatic pressure, and these results were corroborated by RT-PCR. Further, we determined the functional importance of the microRNA most sensitive to hydrostatic pressure, miR-3180-5p. Transfection of HBSMCs with mimics of miR-3180-5p or with a miR-3180-5p inhibitor clearly demonstrated that miR-3180-5p significantly promoted the proliferation of HBSMCs under hydrostatic pressure.
It has been reported that microRNAs can bind to target sites with partial complementation at the 3′ UTR of mRNA and can interfere with mRNA stability or translational efficiency15. We selected podocan, a novel member of the family of small leucine-rich repeat proteins that is commonly found in the extracellular matrix16,17 because proteins in that family are highly effective and selective modulators of important cellular functions18,19,20, including proliferation. Our data indicated that miR-3180-5p directly affects PODN, as observed by decreased PODN mRNA expression levels using RT-PCR and reduced podocan levels in western blots from cells exposed to miR-3180-5p, and this effect was confirmed as a direct effect based on a luciferase reporter assay. Therefore, it was apparent that miR-3180-5p inhibits PODN gene expression, leading to decreased production of its functional protein, podocan. Consistent with our findings, Shimizu-Hirota et al. reported that podocan overexpression is associated with an increase in p21 expression and a decrease in cdk2 expression21. The activation of a cdk2/cyclin complex is involved in the transition from G1 to S phase in the cell cycle, and this complex can be inhibited by p2122. Our flow cytometry results confirmed that the percentage of cells in (S + G2/M) increased when podocan levels were reduced by the overexpression of miR-3180-5p. This result essentially suggests that when miR-3180-5p is overexpressed, transcription of PODN is limited, and the levels of podocan decrease. This leads to the activation of its downstream pathway, the cdk2 signaling pathway, which promotes cellular proliferation. In addition, the changes in PODN, podocan, cdk2 and p21 were confirmed by a complementary approach utilizing a miR-3180-5p inhibitor. In summary, a better understanding of the relationship between microRNAs and proliferative signaling pathways may provide important novel targets for engineering bladder tissue.
However, there remain some limitations of our study. This study was based on overexpression by transfection, which can induce thousand-fold microRNA overexpression but only 7-fold hydrodynamic pressure. However, when the results are compared with previously published reports of cell proliferation under HP, the increase in cell proliferation is similar to that observed in our miR-3180-5p overexpressing transfected cells3. Thus, we can conclude that miR-3180-5p is involved in the HP-induced HBSMC proliferation. The next important point here is that the factors regulating the miR-3180-5p levels remain unknown. A few studies have shown that long noncoding RNAs (lncRNAs) inhibit or promote microRNA expression during cell biological processes23,24. Our preliminary gene-chip results showed that there are some lncRNAs that can target miR-3180-5p. However, this possibility requires further investigation.
Methods
HBSMC culture
HBSMCs (Cat. No. 4310; ScienCell, USA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37 °C in a humidified chamber with 5% CO2. The morphological characteristics of HBSMCs were verified based on the using an optical microscope. Immunofluorescence was used to assess the expression of α-SM actin, the smooth muscle cell marker. Then, experiments were performed using cells between passages 3 and 5 that exhibited normal morphology and good activity.
Hydrostatic pressure
Ex-situ hydrostatic pressure was applied to HBSMCs using a custom-designed motorized pressure apparatus, as previously reported25. In brief, the entire system was placed in a CO2 incubator to ensure constant temperature, atmosphere, and humidity. The HBSMCs were then subjected to cyclic hydrodynamic pressure (HP), simulating the natural bladder cycle (2 h/cycle). In each cycle, the pressure was slowly increased from 0 cm H2O to 10 cm H2O in the first 1.45 hours, then rapidly from 10 cm H2O to 200 cm H2O in the next 0.15 h, with a final pressure drop to 0 cm H2O at the end of the 2-h cycle. Such 2-h cycles were repeated for up to 24 h for HBSMCs, and these cells were referred to as the HP cells. Control HBSMCs were maintained under static conditions (0 cm H2O) in the same atmospheric conditions with computerized pressure monitoring and regulation.
MicroRNA and mRNA microarray
Total RNA was extracted from HP and control cells using TRIzol (Invitrogen, Carlsbad, CA, USA) and miRNeasy mini kits (QIAGEN) according to the manufacturer’s instructions. The analyses of the data were also performed in accordance with the manufacturer’s instructions. The microRNAs and mRNAs showing differential expression in the hydrostatic pressure group and the static control group were identified through fold-change filtering (fold change ≥2.0)26. Target analyses of microRNAs and mRNAs were performed using three tools: Microcosm, miRanda, and TargetScan26. Only those microRNAs and mRNAs for which the target relationship could be established were analyzed further. Gene function prediction was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) categories26. Only those microRNAs that targeted genes inhibiting cell proliferation were chosen for validation.
Real-time PCR
microRNA
Total RNA was extracted using TRIzol, and cDNA was synthesized using an All-in-OneTM miRNA qRT-PCR Detection Kit (GeneCopoeia, Foster City, CA, USA) according to the manufacturer’s instructions. Total microRNA was quantified using a Homo sapiens snRNA U6 qPCR primer as an internal control. The PCR conditions were also programmed according to the manufacturer’s instructions for the All-in-OneTM miRNA qRT-PCR Detection Kit from GeneCopoeia. The sequences were synthesized by GeneCopoeia.
mRNA
Total RNA was extracted using TRIzol, and cDNA was synthesized with an iScriptTM cDNA Synthesis Kit (Bio-Rad, Richmond, CA, USA) using the housekeeping gene GAPDH as an internal control. The PCR cycles were run according to the instructions for SYBR Premix Ex Taq II (TAKARA, Dalian, Liaoning, China). The primer sequences were as follows: (i) ADARB1: 5′-GAAACTCCTGACAAGGCGGA-3′, (ii) NOX4: 5′-GCCAACGAAGGGGTTAAACA-3′, and (iii) PODN: 5′-GCAAAACAACCGCCTGACTT-3′.
These PCRs were performed using a Bio-Rad iQ5 thermal cycler (Hercules, CA, USA), and the quality of the resulting PCR products was monitored via a post-PCR melt curve analysis.
Transfection experiment
To validate the functional significance of the identified microRNAs, we selected the microRNA 3180-5p (miR-3180-5p) because it was found to be upregulated in response to hydrostatic pressure. HBSMCs were transfected with miR-3180-5p mimics or inhibitor at 100 nM (GenePharma, Shanghai, China) using the transfection reagent Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The control HBSMCs were treated with 10 nM non-targeting control sequence (GenePharma, Shanghai, China). Functional assays (flow cytometry and Western blots) were performed 48 h after transfection.
Cell Counting Kit 8
Cell proliferation was assessed using a Cell Counting Kit 8 (CCK-8). The WST-8, which produces water-soluble formazan dye upon bioreduction in the presence of an electron carrier, was included in this system. After transfection, the HBSMCs (5 × 103 cells/100 μl/well) were seeded into a 96-well flat-bottomed plate with culture medium (DMEM with 10% FBS). A blank control was performed using DMEM containing 10% CCK-8. The cells with CCK-8 (10 μl/well) were incubated at 37 °C for 2 h. After cultivation, absorbances at 450 nm (calibrated wave) were measured using a microplate reader (BioTek, Montpelier, VT, USA).
Western blot analysis
At 48 h after transfection, the cells were lysed, and proteins were extracted and pooled using a Nuclear and Cytoplasmic Extraction Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. The extracted proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to western blot analyses using anti-cyclin-dependent kinase (cdk) 2 and anti-p21 and β-actin antibodies (all at 1:500; Abcam, Cambridge, MA, USA). Reactive protein bands were detected by the chemiluminescence method (ECL Plus Western Blot Detection System; Amersham Biosciences, Foster City, CA, USA).
Luciferase assay
Luciferase constructs were synthesized using ligating oligonucleotides containing wild-type or mutant target sites for miR-3180-5p and were inserted into a psiCHECK-2 vector (Promega, Madison, WI, USA). For the luciferase assay, 293T cells (human embryonic kidney cells) were co-transfected with wild-type or mutant psiCHECK-PODN reporter vectors and miR-3180-5p mimics or a non-targeting control mimic sequence (as the NC groups) using Lipofectamine 3000 (Invitrogen). Luciferase activity was measured 48 h post-transfection using a Dual-Luciferase Assay Kit (Promega, Madison, WI, USA).
Flow cytometry
HBSMCs were transfected with miR-3180-5p mimics, a miR-3180-5p inhibitor or a non-targeting control sequence, grown for 48 h, and then washed three times in phosphate-buffered saline (PBS). The cells were centrifuged at 1000 g for 5 min to separate the cells. The supernatant was discarded, and the pellet was fixed in 75% chilled ethanol overnight at 4 °C. Centrifugation was performed again, as described above, and the pellet was washed three times with PBS and re-suspended in 400 ml PBS containing 50 mg/mL RNase A and propidium iodide for 30 min in the dark. After filtration, the cell cycle was examined by flow cytometry using an EPICS ELITE ESP flow cytometer (Beckman Coulter, Miami, FL, USA). The cell proliferation index was calculated using a parameter that reflects the cell proliferation rate, according to the following formula: proliferation index (%) = (S + G2/M)/(G0/G1 + S + G2/M) × 100.
RNA in situ hybridization of miR-3180-5p and PODN
To study the clinical relevance of miR-3180-5p and its target gene PODN, the expression levels of miR-3180-5p and PODN were investigated in patient samples by in situ hybridization. After receiving ethical approval and acquiring informed consent from the patients (patients undergoing radical cystectomy for muscle-invasive bladder cancer), we collected the normal bladders. Then, we isolated tissues from the macroscopically normal areas of the bladders. To verify normality, marginal samples were transferred to the Pathology Department, West China Hospital. Each suitable full-thickness tissue was collected and washed three times with PBS. In addition, we removed the urothelial and serosal layers from the smooth muscle layer. The remaining muscle layer was sheared to a 1 × 1 × 1 cm in size and then expanded in a culture dish.
RNA in situ hybridization for miR-3180-5p and PODN was performed after HBSMCs were subjected to cyclic hydrodynamic pressure. In brief, diethylpyro-carbonate (DEPC) was used to fix the cells, followed by digestion, autofluorescence elimination, blocking of endogenous biotin and degeneration. Next, samples were hybridized overnight with miR-3180-5p probe or PODN probe at 28 °C or 34 °C, respectively. After washing, the samples were blocked with bovine serum albumin (BSA; Solarbio, Beijing, China), and 488-Avidin (1:400; Sigma-Aldrich, Saint Louis, MO, USA) was added. DAPI was used to dye the cell nuclei. Images were captured using an inverted fluorescent microscope (Nikon ECLIPSE 80i, Tokyo, Japan). Image-Pro Plus 6.0 software was utilized to select the same light green used as a unified standard for all positive staining, and then for the analysis of each photo on every positive cumulative optical density.
Statistical Analysis
All data are presented as the mean ± standard deviation (SD) and were analyzed by Student’s unpaired t-tests or by analysis of variance (ANOVA), as appropriate. P values of ≤0.05 were considered to be statistically significant.
Statement about the methods
We confirm that all methods were carried out in accordance with relevant guidelines and regulations. And we confirm that all experimental protocols were approved by Institute of Urology (Laboratory of Reconstructive Urology), West China Hospital, Sichuan University and Ethics Committee of Sichuan University. In addition, we confirming that informed consent was obtained from all subjects.
Conclusion
To our knowledge, this is the first study reporting the involvement of microRNA in cyclic HP-stimulated proliferation of HBSMCs. In this study, we demonstrated that hydrostatic pressure upregulated/downregulated multiple microRNAs in HBSMCs and that the proliferation of HBSMCs could be altered by the overexpression of a single miRNA (miR-3180-5p), which reduces PODN expression, leading to cdk2-mediated proliferation of HBSMCs. Thus, miR-3180-5p may be a novel molecular target for the regeneration of cells in engineering bladder tissue.
Additional Information
How to cite this article: Sun, Y. et al. MiR 3180-5p promotes proliferation in human bladder smooth muscle cell by targeting PODN under hydrodynamic pressure. Sci. Rep. 6, 33042; doi: 10.1038/srep33042 (2016).
References
Karen, M. et al. The effects of sustained hydrostatic pressure on select bladder smooth muscle cell functions. J Urol 162, 2114–2118(1999).
Steers, W. D. Physiology and pharmacology of the urinary bladder. Campbell’s Urology. 7, 875–888 (1998).
Chen, L. et al. Simulated Bladder Pressure Stimulates Human Bladder Smooth Muscle Cell Proliferation via the PI3K/SGK1 Signaling Pathway. J Urol 188, 661–667 (2012).
Wu, T. et al. Effect of cyclic hydrodynamic pressure-induced proliferation of human bladder smooth muscle through Ras-related C3 botulinum toxin substrate 1, mitogen-activated protein kinase kinase 1/2 and extracellular regulated protein kinases 1/2. Int J Urol 19, 867–874 (2012).
Luo, D. Y. et al. Integrin av Mediates Contractility Whereas Integrin a4 Regulates Proliferation of Human Bladder Smooth Muscle Cells via FAK Pathway under Physiological Stretch. J Urol 190, 1421–1429 (2013).
Weber, M. et al. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. BBRC 393, 643–8 (2010).
Ambros, V. The functions of animal microRNAs. Nature. 431, 350–5 (2004).
Chekulaeva, M. & Filipowicz, W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 21, 452–60 (2009).
Lewis, B. P. et al. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 120, 15–20 (2005).
Mohamed, J. S. et al. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3β. J Biol Chem. 285, 29336–47 (2010).
Bhattachariya, A. et al. Stretch-sensitive down-regulation of the miR-144/451 cluster in vascular smooth muscle and its role in AMP-activated protein kinase signaling. PLoS One 8, e65135 (2013).
Ekman, M. et al. MicroRNAs in Bladder Outlet Obstruction: Relationship to Growth and Matrix Remodelling. Basic Clin Pharmacol Toxicol (2015).
Hu, B. et al. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PloS ONE 9, e96338 (2014).
Duan, P. et al. Downregulation of miR-223 and miR-153 mediates mechanical stretch-stimulated proliferation of venous smooth muscle cells via activation of the insulin-like growth factor-1 receptor. Arch Biochem Biophys. 528, 204–211 (2012).
Ghosh, T. et al. MicroRNA-mediated up-regulation of an alternatively polyadenylated variant of the mouse cytoplasmic {beta}-actin gene. Nucleic Acids Res. 36, 6318–6332 (2008).
Hutter, R. et al. The Novel Small Leucine-Rich Repeat Protein Podocan is a Negative Regulator of Migration and Proliferation of Smooth Muscle Cells, Modulates Neointima Formation and is Expressed in Human Atheroma. Circulation. 128, 2351–63 (2013).
Iozzo, R. V. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 274, 18843–6 (1999).
Daley, W. P. et al. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 121, 255–64 (2008).
Dijke, P. & Arthur, H. M. Extracellular control of tgfbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 8, 857–69 (2007).
Raines, E. W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: Relationships to vascular disease. Int J Exp Pathol. 81, 173–82 (2000).
Hirota, R. S. et al. Functional characterization of podocan, a member of a new class in the small leucine-rich repeat protein family. FEBS. 563, 69–74 (2004).
Boehm, M. & Nabel, E. G. Cell cycle and cell migration: new pieces to the puzzle. Circulation. 103, 2879–81 (2001).
Song, B. et al. Long non-coding RNA HOTAIR promotes HLA-G expression via inhibiting miR-152 in gastric cancer cells. BBRC. 46, 807e13 (2015).
Xing, C. Y. et al. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Letters. 589, 1981–1987 (2015).
Stover, J. & Nagatomi, J. Cyclic pressure stimulates DNA synthesis through the PI3K/Akt signaling pathway in rat bladder smooth muscle cells. Annals of biomedical engineering. 35, 1585–1594 (2007).
Fu, Y. R. et al. Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J Cell Mol Med. 18, 503–513 (2014).
Acknowledgements
This work was supported by the National Science Foundation for Young Scholars of China (Grant No. 81300579), the National Natural Science Foundation of China (Grant No. 81470927, 31370951 and 31170907), 135 Project of West China Hospital of Sichuan University, Technology Department of Chengdu City(No. 2014-HM01-00120-SF), Outstanding Youth Foundation of Sichuan University(2014SCU04B21), and Ministry of organization of Sichuan(Grant No. JH2015017).
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of entire study: D.-Y.L., Y.S. and C.T. Studyconcepts: D.-Y.L., Y.S. and Y.-C.Z. Study design: D.-Y.L., L.Z., T.-X.Y., H.S. and K.-J.W. Literature research: D.-Y.L., Y.S. and K.-J.W. Experimental studies: D.-Y.L., L.Z. and T.-X.Y. Data analysis/interpretation: D.-Y.L. and K.-J.W. Statistical analysis: D.-Y.L. and K.-J.W. Manuscript preparation: D.-Y.L. and Y.S. Manuscript definition of intellectual content: D.-Y.L. and Y.S. Manuscript editing: D.-Y.L. and Y.S. Manuscript revision/review: K.-J.W. Manuscript final version approval: D.-Y.L.,Y.S. and K.-J.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, Y., Luo, DY., Zhu, YC. et al. MiR 3180-5p promotes proliferation in human bladder smooth muscle cell by targeting PODN under hydrodynamic pressure. Sci Rep 6, 33042 (2016). https://doi.org/10.1038/srep33042
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33042
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.