Abstract
The role of sphingosine 1-phosphate (S1P) in liver fibrosis or inflammation was not fully examined in human. Controversy exists which S1P receptors, S1P1 and S1P3 vs S1P2, would be importantly involved in its mechanism. To clarify these matters, 80 patients who received liver resection for hepatocellular carcinoma and 9 patients for metastatic liver tumor were enrolled. S1P metabolism was analyzed in background, non-tumorous liver tissue. mRNA levels of sphingosine kinase 1 (SK1) but not SK2 were increased in livers with fibrosis stages 3–4 compared to those with 0–2 and to normal liver. However, S1P was not increased in advanced fibrotic liver, where mRNA levels of S1P transporter spinster homolog 2 (SPNS2) but not S1P-degrading enzymes were enhanced. Furthermore, mRNA levels of S1P2 but not S1P1 or S1P3 were increased in advanced fibrotic liver. These increased mRNA levels of SK1, SPNS2 and S1P2 in fibrotic liver were correlated with α-smooth muscle actin mRNA levels in liver, and with serum ALT levels. In conclusion, S1P may be actively generated, transported to outside the cells, and bind to its specific receptor in human liver to play a role in fibrosis or inflammation. Altered S1P metabolism in fibrotic liver may be their therapeutic target.
Similar content being viewed by others
Introduction
Liver fibrosis results from chronic liver injury, and is characterized by excessive deposition of collagen and other components of extracellular matrix. Although the exact mechanism of liver fibrosis still remains to be clarified, sphingosine 1-phosphate (S1P) is now assumed to be among pivotal regulators of liver fibrosis1. Many biological actions of S1P as an extracellular mediator are generated through binding to G-protein-coupled S1P receptors 1–5 2. S1P1-3 are widely expressed in a variety of tissues including liver, whereas S1P4 is exclusively found on lymphoid and hematopoietic tissue and S1P5 is mainly expressed in the central nervous system3. To bind S1P receptors, a large amount of S1P may be secreted to the extracellular space, where the export of S1P from the cells requires the action of S1P transporter spinster homolog 2 (SPNS2)4,5,6,7. To generate S1P inside the cells, sphingosine kinases (SKs) are known to be a key enzyme with two isoforms (SK1 and SK2), differing in sequence, catalytic properties, localization, and functions8.
Hepatic stellate cells (HSCs) are known to play a central role in the development of liver fibrosis, undergoing phenotypic change to myofibroblast-like cells in the process of liver fibrosis9,10,11. We first found that S1P stimulated rat HSC proliferation via an extracellular mechanism in vitro12, and later we further observed that liver fibrosis caused by repeated administration of carbon tetrachloride was reduced in S1P receptor 2-deficient mice in vivo13. Moreover, S1P has a stimulatory role in portal hypertension14, where S1P2 antagonist is effective in reducing portal vein pressure in rodents15. In contrast, an important role of S1P1 and S1P3 but not S1P2 in liver fibrosis has been demonstrated in mice16. Furthermore, the contribution of intracellular S1P to liver fibrosis, independent of its receptors, has been recently reported in human17. Although these lines of evidence suggest that S1P plays an important role in pathophysiology of liver fibrosis, the contribution of its receptors to liver fibrosis remains elusive. Furthermore, the mode of action of S1P in liver fibrosis may vary with species examined. If so, a role of S1P in liver fibrosis should be examined in human in order to establish therapeutic strategy for liver fibrosis by regulating the effect of S1P. In the current study, we sought to examine the expressions of S1P and its metabolites and receptors, and the enzymes in its metabolic pathway in human liver tissue with various stages of fibrosis.
Results
Patient characteristics
A total of 80 hepatocellular carcinoma (HCC) patients who underwent a surgical treatment were enrolled. Clinical and laboratory characteristics of the patients are shown in Table 1. There were 61 male and 19 female patients with a median age of 71 years. Fourteen patients (17.5%) were with hepatitis B virus infection, and 32 patients (40.0%) with hepatitis C virus infection. Seventy-eight (97.5%) of the patients was with Child-Pugh class A, and fibrosis stage 4 in the background liver was observed in 26 patients (32.5%).
Alterations of sphingolipid metabolites and the related enzymes in human fibrotic liver
Table 2 shows the associations between histological fibrosis stage and mRNA expressions or levels of sphingolipid metabolites and the related enzymes in the background, non-tumorous liver tissues. SK1 mRNA expression levels in liver were significantly higher in patients with advanced liver fibrosis (P = 0.005, after adjustment for age and sex); the median mRNA expression level of SK1 in livers with fibrosis stages 0–2 and 3–4 were 1.79 × 1/105 (1.21 × 1/105–3.41 × 1/105) and 3.67 × 1/105 (2.02 × 1/105–6.63 × 1/105), respectively. In contrast, SK2 mRNA expression levels were not different between livers with fibrosis stages 0–2 and those with 3–4. In spite of the increased mRNA levels of SK1, catalyzing the synthesis of S1P from sphingosine, S1P levels as well as Sph levels were not different between livers with fibrosis stages 0–2 and those with 3–4. Then, regarding a degrading enzyme of S1P, both S1P lyase (SPL) and S1P phosphatase 1 (SPP1) mRNA expression levels were not different between livers with fibrosis stages 0–2 and those with 3–4, while mRNA expression levels of SPNS2, a transporter of S1P from inside the cells to outside, were significantly higher in livers with fibrosis stages 3–4 than in those with 0–2 (P = 0.03, after adjustment for age and sex); the median mRNA expression level of SPNS2 in livers with fibrosis stages 0–2 and 3–4 were 1.84 × 1/105 (1.43 × 1/105–2.54 × 1/105) and 2.38 × 1/105 (1.89 × 1/105–3.68 × 1/105), respectively. Among S1P receptors, S1P2 mRNA expression levels were higher in livers with fibrosis stages 3–4 than in those with 0–2 (P = 0.04, univariate analysis), while S1P1 and S1P3 mRNA levels were not different between livers with fibrosis stages 0–2 and those with 3–4. Collectively, our analysis of mRNA levels showed increased expression of SK1, SPNS2 and S1P2 in livers with fibrotic stages 3–4 compared to those with 0–2, and the similar trend to mRNA levels was observed in protein levels of SK1, SPNS2 and S1P2 analyzed by Western blot using the residual liver tissues (Supplementary Figure 1). These results suggest that inside-out signaling18 of S1P may operate actively in fibrotic liver, and in line with this a strong correlation between SPNS2 and S1P2 mRNA levels in liver was observed (Spearman’s rho = 0.6057, P < 0.001, data not shown). We also investigated the associations between histological fibrosis stages and mRNA expressions or levels of sphingolipid metabolites and the related enzymes in patients with hepatitis B, with hepatitis C, or without hepatitis viruses (Supplementary Tables 1–3). Although statistically significant differences were not observed, SK1, SPNS2, and S1P2 mRNA levels were higher in patients with fibrosis stages 3–4 compared to 0–2 in all etiologies (Supplementary Figure 2).
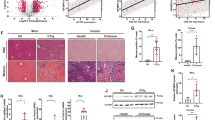
Then, to investigate whether the alterations of sphingolipid metabolites and the related enzymes in fibrotic liver might be associated with HSCs as a central player in liver fibrosis, we measured mRNA levels of α-smooth muscle actin (α-SMA), a marker of HSCs with phenotypic change to myofibroblast-like cells. As depicted in Fig. 1, mRNA levels of α-SMA were significantly correlated with Sph, S1P and dhS1P levels, and mRNA levels of SK1, SK2, SPL, SPP1 or SPNS2 in liver. Furthermore, mRNA levels of S1P1, S1P2, and S1P3 were significantly correlated with α-SMA mRNA levels in liver (Fig. 1F–H). Collectively, there may be a link between the altered S1P metabolism in human fibrotic liver and HSCs.
Relationship between α-SMA mRNA expression and mRNA expressions (or levels) of Sph (A), S1P (B), dhS1P (C), SK1 (D) SK2 (E), SPL (F), SPP (G), SPNS2 (H), S1P1 (I), S1P2 (J), and S1P3 (K) in liver tissue. A significant correlations were observed between α-SMA mRNA expression and mRNA expression/levels of Sph (spearman’s rank correlation coefficient; ρ = 0.57, P < 0.001), S1P (spearman’s rank correlation coefficient; ρ = 0.34, P = 0.003), dhS1P (spearman’s rank correlation coefficient; ρ = 0.41, P < 0.001), SK1 (spearman’s rank correlation coefficient; ρ = 0.60, P < 0.001), SK2 (spearman’s rank correlation coefficient; ρ = 0.62, P < 0.001), SPL (spearman’s rank correlation coefficient; ρ = 0.46, P < 0.001), SPP1 (spearman’s rank correlation coefficient; ρ = 0.31, P = 0.006), SPNS2 (spearman’s rank correlation coefficient; ρ = 0.51, P < 0.001), S1P1 (spearman’s rank correlation coefficient; ρ = 0.68, P < 0.001), S1P2 (spearman’s rank correlation coefficient; ρ = 0.58, P < 0.001), or S1P3 (spearman’s rank correlation coefficient; ρ = 0.60, P < 0.001).
Finally, in order to compare normal liver with advanced fibrotic liver in human from the viewpoint of sphingolipid metabolites and the related enzymes, we have obtained normal liver samples from the patients undergoing hepatic resection for metastasis from colorectal cancer, although the number of patients was small (n = 9). The clinical and laboratory characteristics of these patients are shown in the Supplementary Table 4, and mRNA expression or levels of sphingolipid metabolites and the related enzymes in normal liver and advanced fibrotic liver are shown in the Supplementary Table 5. In line with the comparison between liver fibrosis stages 0–2 and 3–4, the higher SK1, SPNS2, and S1P2 mRNA levels were noted in the patients with fibrosis stages 3–4 compared to the patients with normal liver, though not statistically significant due to the relatively small number of patients with normal liver.
Relationships of sphingolipid metabolites and the related enzymes in human fibrotic liver with clinical parameters
Then, the relationships between sphingolipid metabolites and the related enzymes in human fibrotic liver and clinical parameters were examined. Table 3 shows the relationship of sphingosine metabolites and the related enzymes with clinical parameters. There was no association between S1P levels in liver and clinical parameters. On the other hand, among S1P-related enzymes in liver, SK1 mRNA levels were correlated with serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin levels, SK2 with serum γ-glutamyl transpeptidase (GGT) levels, SPL, SPP1 and SPNS2 with serum ALT levels. Furthermore, S1P1, S1P2 and S1P3 mRNA levels in liver were correlated with serum ALT levels.
Regarding the etiology of liver diseases, SPL and SPNS2 mRNA levels in liver were lower in the patients with hepatitis B virus infection than in those with other etiology (data not shown).
Discussion
Although S1P has been shown to play an important role in liver fibrosis or inflammation in rodents1,19,20, the clinical roles of S1P and its metabolites in liver fibrosis or inflammation have not been fully elucidated in human. Here, we examined the relationship between the severity of liver fibrosis/inflammation and mRNA expressions and/or levels of sphingolipid metabolites and the related enzymes using surgically-resected human liver tissues. In the current study, mRNA expression of SK1 but not SK2 was enhanced in fibrotic liver in human, in line with the previous report21, however, S1P, generated with catalytic action of SK1, was not increased in human fibrotic liver. There were at least two possibilities to explain these findings, increased degradation and/or export of S1P. As a result, mRNA expression of SPL or SPP1, an enzyme to degrade S1P, was not altered, while mRNA expression of SPNS2, a transporter of S1P, was enhanced in human fibrotic liver. Furthermore, mRNA expression of S1P2 among S1P receptors was enhanced in fibrotic liver in human. These results suggest that S1P, generated actively by SK1 in human fibrotic liver, may be transported to the outside of liver cells by SPNS2, and that the exported S1P may bind to S1P2 to exert its action by an extracellular mechanism.
Controversy exits regarding the contribution of S1P receptors to liver fibrosis. The enhanced mRNA expression of S1P2 has been previously reported in fibrotic liver in rodents15, while S1P2 expression were not altered in liver fibrosis in rodents16 but massively decreased in human21. The current finding in human is in line with the former evidence in rodents, which showed up-regulation of S1P2 in fibrotic liver, and may suggest a rationale to establish therapeutic strategy for liver fibrosis by modifying S1P action based on the evidence in rodents. In this regard, we13 and others19,22 have reported an important role of S1P/S1P2 axis in liver fibrosis using S1P2-deficient mice or S1P2 antagonist in rodents. Thus, antagonism of S1P2 merits consideration as therapeutic strategy for liver fibrosis in human. On the other hand, the overexpression of S1P1 and S1P3 has been reported in human fibrotic liver tissue21. The reasons of these discrepancies have not been solved yet, and further studies with more samples may be useful to find a precise role of S1P receptors in liver fibrosis.
To the best of our knowledge, this is the first report showing the altered expression of SPNS2 in liver. It should be further elucidated whether enhanced expression of SPNS2 in liver might be simply associated with liver fibrosis or importantly involved in its mechanism. Although a gain- and loss-of-function approach may be useful to clarify how SPNS2 sustains fibrogenic process in liver, no phenotype has been reported in liver in SPNS2-knockout mice so far7. Thus, a role of SPNS2 in liver fibrosis should be further elucidated. Of interest is a fact that a mutation in SPNS2 caused similar phenotype to that in S1P2, a defect in heart development in zebrafish4. Furthermore, SPNS2 deficiency23 and S1P2 deficiency24 caused similarly hearing loss in mice. These lines of evidence may suggest a crucial link between SPNS2 and S1P2, when S1P exerts its effect. A strong correlation between mRNA levels of SPNS2 and S1P2 in fibrotic liver observed in the current study may be in line with this speculation.
We have recently shown in HCC, which frequently develops in fibrotic liver, the increased mRNA levels in SK1, SK2 and SPL but not altered mRNA levels in SPNS2 and the rather reduced levels of S1P compared to non-HCC tissues25. These findings and the current ones may suggest active inside-out signaling of S1P in fibrotic liver, while enhanced degradation of S1P in HCC, among which crucial events involved in fibrogenesis and carcinogenesis in liver should be further elucidated.
We previously reported that plasma S1P levels were reduced in patients with chronic hepatitis C in association with liver fibrosis26, which may not be consistent with the current results. Although the local concentration of S1P in the outside of liver cells of fibrotic liver may be increased with enhanced expressions of SK1 and SPNS2, the exported S1P by inside-out signaling18 may be bound to S1P receptors and taken up by liver cells, leading to reduced S1P levels in blood stream.
The SK1/S1P pathway has been reportedly implicated also in inflammation mediated by TNF-α27. Other inflammatory signaling molecules such as IFN-λ, IL-1β, IgE28 have been also shown to activate SK1, further suggesting the importance of the SK1/S1P pathway in the inflammatory response. In another study, SK1-knockout mice had decreased tissue SK activity and a 50% decrease in serum S1P levels, but not significant difference in tissue S1P under normal conditions29. In the present study, in spite of positive correlation between serum ALT levels and SK1/SPNS2/S1PRs expressions, serum ALT levels were not correlated with S1P/dhS1P levels in liver tissue. Taken together the above studies and the present study, inflammation and recruitment of inflammatory cells are important in liver disease progression and are, at least in part, mediated by the SK1 and extracellular secretion of S1P via SPNS2. Although higher SK1 expression results in increased S1P concentration in liver cells, intracellular S1P may be reduced by up-regulated SPNS2; and a larger amount of S1P may be secreted to extracellular space to bind S1PRs in patients with active inflammation.
Among the enzymes in the S1P metabolic pathway, acidic sphingomyelinase to generate ceramide was reportedly associated with HSC activation, and its mRNA levels were enhanced in liver samples from patients with nonalcoholic steatohepatitis compared in those from healthy subjects30. On the other hand, acidic sphingomyelinase mRNA levels were not significantly enhanced in fibrotic liver in the current study (data not shown). Collectively, it is speculated that acidic sphingomyelinase mRNA expression in liver might be stimulated by steatosis rather than fibrosis. Nonetheless, a role of acidic sphingomyelinase in liver fibrosis should be further elucidated.
Some limitations of this study should be acknowledged. Firstly, because we used background liver samples of HCC patients or patients with liver metastasis, the current results may be affected by the presence of HCC31 or other liver tumors. However, it is difficult due to the ethical concerns to obtain liver specimen from patients without HCC or other liver tumors, because the vast majority of patients who undergo liver resection are HCC patients or patients with liver metastasis in our hospital. As the second concern, our results may be affected by hepatic decompensation32. However, its effect may be minimized in the present study, because almost all (97.5%) patients were assumed to have well preserved liver function (Child-Pugh classification A). Thirdly, SPL and SPNS2 mRNA levels in liver were lower in the patients with HBV infection than in those with other etiology in the present study, suggesting that it would be interesting to analyze the samples separately according to the etiology of liver diseases. The other studies reported difference of serum sphingolipid profiles among patients with hepatitis C, hepatitis B, and non alcoholic fatty liver disease33,34. In the results of separate analyses according to etiologies (Supplementary Tables 1–3), almost the same tendencies as the results in whole patients were observed in all etiologies (Supplementary Figure 2). However, the sample size in each etiology was relatively small, being statistically underpowered. Further studies with larger samples using liver tissue without HCC such as liver biopsy samples may be worth trying.
In conclusion, our study, using human liver tissue samples, shows that increased activity of SK1/S1P pathway, S1P secretion via SPNS2, and S1P binding to S1P2 may play an important role in the progression of liver fibrosis with recruitment of inflammatory cells. Altered S1P metabolism in fibrotic liver may be a possible drug target for patients with chronic hepatitis or liver cirrhosis.
Patients and Methods
Subjects
The liver tissue samples analyzed in the present study were derived from the patients who were treated in the Hepatobiliary Pancreatic Surgery Division, Department of Surgery, the University of Tokyo Hospital between January 2013 and October 2014. Among them, sufficient liver tissue samples could be obtained in 80 patients to analyze the levels of sphingolipid metabolites and the related enzymes. All the enrolled patients underwent liver resection for the treatment of HCC. Additionally, we enrolled the patients undergoing hepatic resection for metastasis from colorectal cancer in above period to obtain normal liver samples (n = 9).
This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Research Ethics Committee of the University of Tokyo. A written informed consent was obtained for the use of the samples.
Quantitative real time PCR for enzymes in sphingolipid metabolism
Total RNA of tissue samples was extracted using TRIZOL reagent (Invitrogen, CA, USA). One microgram of purified total RNA was transcribed using a SuperScriptTM First-Strand Synthesis System for RT-PCR (Invitrogen). Quantitative real time PCR was performed with a LightCycler FastStart DNA Master SYBR Green I kit (Roche Molecular Diagnostics, CA, USA) or TaqMan Universal Master Mix. The primer pairs used were as follows: human SK1: 5′-CTGGCAGCTTCCTTGAACCAT-3′ and 5′-TGTGCAGAGACAGCAGGTTCA-3′; human SK2: 5′-CCAGTGTTGGAGAGCTGAAGGT-3′ and 5′-GTCCATTCATCTGCTGGTCCTC-3′; human SPL: 5′-GCCAGAGAGTTTATGGTCAAGGTT-3′ and 5′-CAACTTGTCTTGAATCTTACGACCAA-3′; human SPP1: 5′-GCCGCTGGCAGTACCCT-3′ and 5′-AATAGAGTGCATTCCCATGTAAATTCT-3′; internal control ribosomal 18s: 5′-GTAACCCGTTGAACCCCATT-3′ and 5′-CCATCCAATCGGTAGTAGCG-3′; human S1P receptor 1 (S1P1): 5′-CCTCTTCCTGCTAATCAGCG-3′ and 5′-ACAGGTCTTCACCTTGCAGC-3′; human S1P receptor 2 (S1P2): 5′-GCCTAGCCAGTTCTGAAAGC-3′ and 5′-ACAGGTACATTGCCGAGTGG-3′; human S1P receptor 3 (S1P3): 5′-CGACGGAGGAGCCCTTTTTC-3′ and 5′-TCCAAAATCCACGAGAGGGC-3′; acid sphingomyelinase: 5′-AAGCCCTGCGCACCCTCAGAA-3′ and 5′-CCTGAAGCTCCCCCACCAGCC-3′; α-SMA: 5′-CAGCCAAGCACTGTCAGGAAT-3′ and 5′-TTTGCTCTGTGCTTCGTCAC-3′. The samples were incubated for 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 sec, 60 °C for 1 min. The target gene mRNA expression level was relatively quantified to ribosomal 18 s using 2−ΔΔCt method (Applied Biosystems, User Bulletin No. 2).
Measurement of sphingolipids in liver tissues
Sample preparation procedure was referenced by previously reported35. Liver (approximately 10–50 mg) was placed into a sample tube (2.0 mL) and snap frozen in liquid nitrogen. Two-hundred microliters of formic acid (0.1% (volume fraction) in methanol) containing the internal standard solution (10.0 ng/mL, mixture of sphingosine (Sph) 17:1 and S1P 17:1) was added to the frozen sample. The mixtures were homogenized using a lysis and homogenization system (Precellys) (5,000 × rpm, 15 s, 1 Zr bead). The samples were then centrifuged at 16,400 × g for 20 min at 4 °C, and the supernatants were passed through a 96-well plate for deproteinization (Sirocco, Waters). Aliquots (5 μL) of the deproteinized samples were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS system consists of a NANOSPACE SI-II HPLC (Shiseido) and a TSQ Quantum Ultra triple quadrupole MS (Thermo Fisher Scientific) equipped with a heated-electrospray ionization-II source. Sphingolipids were analyzed by LC-MS/MS in the selected reaction monitoring mode, and the optimal condition was described elsewhere25. The ratio of the peak area of the analyte to the internal standard was used for quantification with the calibration curves (0.05–100 ng/mL) by each standard solutions.
Immunoblot analysis
Tissue extracts were prepared by using M-PER® Mammalian Protein Extraction Reagent (Thermo Fisher Scientific Inc., IL, USA) plus HaltTM Protease Inhibitor Cocktail (Thermo Fisher Scientific Inc.). Immunoblot analysis was performed using NuPAGE SDS-PAGE Gel (Invitrogen) and iBlot Dry Blotting System (Invitrogen) with specific antibodies against SK1 (Cell Signaling Technology, MA, USA) dilution 1:1000, SPNS2 (Abcam, Cambridge, UK) dilution 1:150, S1P2 (Novusbio, Minneapolis, USA) dilution 1ug/ml and beta-actin (Sigma-Aldrich, St. Louis Missouri, USA) dilution 1:2000. Immunoreactive proteins were visualized using a chemiluminescence kit (GE Healthcare, Buckinghamshire, UK), and recorded using a LAS-4000 image analyzer (Fuji Film, Tokyo, Japan).
Study end-points
We analyzed the relationship between histological fibrosis stages and mRNA expressions/levels of sphingolipid metabolites and the related enzymes in liver tissues. We also examined the relationship between sphingolipid metabolites and the related enzymes and serological or other biological markers. The histological stages of fibrosis were assessed using the reproducible METAVIR scoring system36,37.
Statistical analysis
Continuous variables were presented as median with 1st and 3rd percentiles while categorical variables were expressed as frequencies (%). For comparison between two groups, Mann-Whitney U test was used; and for comparison of three groups, Kruskal-Wallis test was used. Multiple linear regression model analyses were used to adjust the contribution of fibrosis by other covariates such as sex or age. The potential associations between mRNA expressions and following factors were assessed using Spearman’s rank correlation coefficient: age, platelet count, serum albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin, prothrombin time (PT), and mRNA level of α-smooth muscle actin (α-SMA). All statistical analyses were two-sided, and the threshold of the reported P values for significance was accepted as <0.05. All statistical analyses were performed using R statistic software version 3.1.0 (http://www.r-project.org).
Additional Information
How to cite this article: Sato, M. et al. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Sci. Rep. 6, 32119; doi: 10.1038/srep32119 (2016).
References
Takuwa, Y., Ikeda, H., Okamoto, Y., Takuwa, N. & Yoshioka, K. Sphingosine-1-phosphate as a mediator involved in development of fibrotic diseases. Biochimica et biophysica acta 1831, 185–192, doi: 10.1016/j.bbalip.2012.06.008 (2013).
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews. Molecular cell biology 4, 397–407, doi: 10.1038/nrm1103 (2003).
Sanchez, T. & Hla, T. Structural and functional characteristics of S1P receptors. Journal of cellular biochemistry 92, 913–922, doi: 10.1002/jcb.20127 (2004).
Kawahara, A. et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science (New York, N.Y.) 323, 524–527, doi: 10.1126/science.1167449 (2009).
Hisano, Y., Kobayashi, N., Kawahara, A., Yamaguchi, A. & Nishi, T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. The Journal of biological chemistry 286, 1758–1766, doi: 10.1074/jbc.M110.171116 (2011).
Hisano, Y., Kobayashi, N., Yamaguchi, A. & Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PloS one 7, e38941, doi: 10.1371/journal.pone.0038941 (2012).
Fukuhara, S. et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. The Journal of clinical investigation 122, 1416–1426, doi: 10.1172/jci60746 (2012).
Maceyka, M. et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. The Journal of biological chemistry 280, 37118–37129, doi: 10.1074/jbc.M502207200 (2005).
Friedman, S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 275, 2247–2250. (2000).
Friedman, S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669, doi: 10.1053/j.gastro.2008.03.003 (2008).
Bataller, R. & Brenner, D. A. Liver fibrosis. The Journal of clinical investigation 115, 209–218, doi: 10.1172/jci24282 (2005).
Ikeda, H. et al. Biological activities of novel lipid mediator sphingosine 1-phosphate in rat hepatic stellate cells. American journal of physiology. Gastrointestinal and liver physiology 279, G304–G310 (2000).
Ikeda, H. et al. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. Journal of lipid research 50, 556–564, doi: 10.1194/jlr.M800496-JLR200 (2009).
Ikeda, H. et al. Sphingosine 1-phosphate enhances portal pressure in isolated perfused liver via S1P2 with Rho activation. Biochemical and biophysical research communications 320, 754–759, doi: 10.1016/j.bbrc.2004.04.207 (2004).
Kageyama, Y. et al. Antagonism of sphingosine 1-phosphate receptor 2 causes a selective reduction of portal vein pressure in bile duct-ligated rodents. Hepatology (Baltimore, Md.) 56, 1427–1438, doi: 10.1002/hep.25780 (2012).
Li, C. et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. Journal of hepatology 50, 1174–1183, doi: 10.1016/j.jhep.2009.01.028 (2009).
Xiu, L. et al. Intracellular sphingosine 1-phosphate contributes to collagen expression of hepatic myofibroblasts in human liver fibrosis independent of its receptors. The American journal of pathology 185, 387–398, doi: 10.1016/j.ajpath.2014.09.023 (2015).
Takabe, K., Paugh, S. W., Milstien, S. & Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacological reviews 60, 181–195, doi: 10.1124/pr.107.07113 (2008).
Serriere-Lanneau, V. et al. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 21, 2005–2013, doi: 10.1096/fj.06-6889com (2007).
Liu, Q. et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. Plos one 7, e41834, doi: 10.1371/journal.pone.0041834 (2012).
Li, C. et al. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. Journal of hepatology 54, 1205–1213, doi: 10.1016/j.jhep.2010.08.028 (2011).
Wang, R. et al. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. The Journal of biological chemistry 290, 30684–30696, doi: 10.1074/jbc.M115.671735 (2015).
Chen, J. et al. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. Plos genetics 10, e1004688, doi: 10.1371/journal.pgen.1004688 (2014).
Kono, M. et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. The Journal of biological chemistry 282, 10690–10696, doi: 10.1074/jbc.M700370200 (2007).
Uranbileg, B. et al. Increased mRNA Levels of Sphingosine Kinases and S1P Lyase and Reduced Levels of S1P Were Observed in Hepatocellular Carcinoma in Association with Poorer Differentiation and Earlier Recurrence. Plos one 11, e0149462, doi: 10.1371/journal.pone.0149462 (2016).
Ikeda, H. et al. Plasma concentration of bioactive lipid mediator sphingosine 1-phosphate is reduced in patients with chronic hepatitis C. Clinica chimica acta; international journal of clinical chemistry 411, 765–770, doi: 10.1016/j.cca.2010.02.063 (2010).
Xia, P. et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proceedings of the National Academy of Sciences of the United States of America 95, 14196–14201 (1998).
Alvarez, S. E., Milstien, S. & Spiegel, S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends in endocrinology and metabolism: TEM 18, 300–307, doi: 10.1016/j.tem.2007.07.005 (2007).
Allende, M. L. et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. The Journal of biological chemistry 279, 52487–52492, doi: 10.1074/jbc.M406512200 (2004).
Moles, A. et al. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. The American journal of pathology 177, 1214–1224, doi: 10.2353/ajpath.2010.091257 (2010).
Grammatikos, G. et al. Serum sphingolipidomic analyses reveal an upregulation of C16- ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget, doi: 10.18632/oncotarget.7741 (2016).
Grammatikos, G. et al. Serum Sphingolipid Variations Associate with Hepatic Decompensation and Survival in Patients with Cirrhosis. Plos one 10, e0138130, doi: 10.1371/journal.pone.0138130 (2015).
Grammatikos, G. et al. Variations in serum sphingolipid levels associate with liver fibrosis progression and poor treatment outcome in hepatitis C virus but not hepatitis B virus infection. Hepatology (Baltimore, Md.) 61, 812–822, doi: 10.1002/hep.27587 (2015).
Grammatikos, G. et al. Serum acid sphingomyelinase is upregulated in chronic hepatitis C infection and non alcoholic fatty liver disease. Biochimica et biophysica acta 1841, 1012–1020, doi: 10.1016/j.bbalip.2014.04.007 (2014).
Okudaira, M. et al. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. Journal of lipid research 55, 2178–2192, doi: 10.1194/jlr.D048439 (2014).
Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology (Baltimore, Md.) 20, 15–20 (1994).
Bedossa, P. & Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology (Baltimore, Md.) 24, 289–293, doi: 10.1002/hep.510240201 (1996).
Acknowledgements
This work was supported by CREST from JST/AMED, Grant-in-Aid for Scientific Research on Innovative Areas 15H05906 (to YY) and Health Sciences Research Grants of The Ministry of Health, Labour and Welfare of Japan, Research on Hepatitis, (to MS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.S. statistical analysis, acquisition of data, and drafting of the manuscript H.I.: study concept and design, analysis and interpretation of data, and drafting the manuscript B.U.: acquisition of data, and analysis and interpretation of data M.K.: analysis and interpretation of data D.S., H.K., H.M. and K.H.: acquisition of data J.A. and N.K.: study supervision Y.Y.: study concept and design, and the drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sato, M., Ikeda, H., Uranbileg, B. et al. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Sci Rep 6, 32119 (2016). https://doi.org/10.1038/srep32119
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32119
This article is cited by
-
The Sphingosine 1-Phosphate Axis: an Emerging Therapeutic Opportunity for Endometriosis
Reproductive Sciences (2023)
-
Integrative roles of sphingosine kinase in liver pathophysiology
Toxicological Research (2023)
-
Candidate lncRNA–miRNA–mRNA network in predicting hepatocarcinogenesis with cirrhosis: an integrated bioinformatics analysis
Journal of Cancer Research and Clinical Oncology (2020)
-
Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo
Scientific Reports (2017)
-
Tricyclic Antidepressants Promote Ceramide Accumulation to Regulate Collagen Production in Human Hepatic Stellate Cells
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.