Abstract
CD4+CD25+ T cells are critical for maintenance of immunologic self-tolerance. We measured the number of CD4+CD25+ cells in the patients with primary malignant hypertension related kidney injury, to explore the molecular pathogenesis of this disease. We selected 30 patients with primary malignant hypertension related kidney injury and 30 healthy volunteers. Information on clinical characteristics and laboratory tests was obtained from each subject. The number of CD4+CD25+ cells and glomerular injury were assessed by flow cytometry and histopathology, respectively. Both serum IL-2, IL-4, and IL-6 and endothelial cell markers were analyzed by ELISA. ADAMTS13 antibody was detected by Western blotting. CD4+CD25+ cells were significantly reduced in patients with primary malignant hypertension related kidney injury compared to controls (P < 0.05). The number of CD4+CD25+ cells was negatively related to blood urea nitrogen, serum uric acid, proteinuria, and supernatant IL-4; whereas positively associated with estimated glomerular filtration rate in patients. Gradually decreasing CD4+CD25+ cells were also found as increasing renal injury. Additionally, patients exhibited increasing supernatant IL-4, serum IL-2 and IL-6, endothelial cell markers, and anti-ADAMTS13 antibody compared with controls (all P < 0.05). CD4+CD25+ cells may play a key role in the pathogenesis of primary malignant hypertension related kidney injury.
Similar content being viewed by others
Introduction
Malignant hypertension (MHTN) is defined as severely elevated blood pressure (BP) (diastolic BP > 130 mm Hg) with a Keith and Wagener stage III or IV retinopathy1. MHTN is a rare but serious syndrome accounting for 1% of essential hypertension, which can cause neurological, renal and cardiac complications. Despite improved anti-hypertensive medication, the incidence of MHTN fails to decline2. MHTN is characterized by severe hypertension and acute multi-organ ischemic complications including thrombotic microangiopathy (TMA). Renal TMA accompanying MHTN is due to thrombosis of small vessels, intravascular hemolysis with red cell fragments (Schistocytes), and platelet consumption3.
The vascular injury observed in MHTN is unique, where fibrinoid necrosis and proliferative endarteritis affect resistance vessels. Fibrinoid necrosis is defined as vascular smooth muscle cell death with deposition of plasma proteins (e.g., fibrin). Proliferative endarteritis is defined as chronic endarteritis accompanied by a marked increase of fibrous tissue in the inner lining of the artery, characterized by myointimal proliferation with luminal narrowing. However, the triggers and molecular mechanisms involved in these vessel pathologies remain unclear, although several reports indicate the involvement of inflammatory mediators4,5.
Recent studies have shown that CD4+CD25+ cells display a crucial role in the immunological self-tolerance via inhibiting the proliferation and activation of autoreactive T cells6. Their depletion might result in the organ-specific autoimmunity developing and autoimmunity diseases, and these changes could be prevented by CD4+CD25+ cells in the animal models7. Powrie and colleagues8 demonstrated that transfer of CD4+CD25+ cells could not only protect the mice from developing inflammatory bowel disease, but also reverse the established gastrointestinal inflammation. Until now, CD4+CD25+ cells have shown to be regulators in almost all of allergic diseases, transplant rejection, and animal models with human organ specific diseases9.
Regulatory T lymphocytes (Tregs) can regulate or suppress the function of other immune cells. CD4+CD25+ cells, a type of Tregs, have been identified in mice and humans as a distinct population of CD4+ T cells that constitutively express the interleukin (IL)-2 receptor α-chain (CD25)10,11. CD4+CD25+ cells have been shown to dampen local anti-tumor responses and to prevent sterilizing immunity against certain chronic infectious agents. CD4+CD25+ cells occasionally mediate peripheral tolerance, leading to an exacerbated inflammatory/allergic reaction or autoimmunity12.
To date, there are very few studies of CD4+CD25+ cells in human MHTN related kidney injury. Here, we investigated the association between the number change of CD4+CD25+ cells and the development of MHTN related kidney injury using a case-control study to test the hypothesis that a numerical or functional deficit of CD4+CD25+ cells might trigger the development of disease.
Methods
Subjects
Thirty patients with MHTN related kidney injury were included in this study. The patients were diagnosed with clinically and renal biopsy confirmed TMA, hospitalized in Hunan Provincial People’s Hospital and Second Xiangya Hospital of Central South University from January 2006 to February 2014. The patients included 20 men and 10 women, aged 25–57 years (mean ± SD: 42.5 ± 8.61 years). All patients met the criteria for MHTN related kidney injury13. Kidney injury was defined as renal damages and/or functional impairment. Secondary MHTN (e.g., renovascular hypertension, primary hyperaldosteronism, pheochromocytoma, polyarteritis nodosa, and renal parenchymal disorders) was excluded. Other factors that could lead to TMA, including cyclosporine A toxicity, liver cirrhosis, cancer, and postpartum renal failure, were also excluded.
Thirty healthy volunteers including 19 men and 11 women, age 30–56 years; (mean ± SD: 43.7 ± 9.65 years) were selected as controls. Patients and controls were all Chinese Han and matched by age and gender.
The present study was approved by the Ethical Committee of Hunan Provincial People’s Hospital and the Second Xiang-ya Hospital of Central South University. All the experiments were carried out in accordance with the approved guidelines and regulations. Each subject had signed written informed consent.
Calculation of estimated glomerular filtration rate (eGFR)
Renal function was assessed by eGFR, which was calculated using CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation14. eGFR = 141 X min(Scr/κ,1)α X max(Scr/κ,1)−1.209 × 0.993Age X 1.018 [if female], where Scr. is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, respectively, α is −0.329 for females and −0.411 for males, respectively, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
Data Collection
After informed consent was obtained, peripheral blood and urine samples and clinical data were collected. None of the patients had undergone treatment with angiotensin converting enzyme (ACE) inhibitor, immunosuppressive agents, steroid, or AT1 receptor blockers before clinical samples collection. The laboratory examinations included urinalysis, complete blood count, serum chemistries, endothelial markers [von Willebrand factor (vWF), vascular cell adhesion molecule (VCAM)], and immunologic serologic index (anti-nuclear antibody, anti–double-stranded DNA antibody, anti-neutrophil cytoplasmic antibody, anti -endothelial cell antibody, and complement components C3).
Flow cytometry
The live-dead lymphocytes and CD4+CD25+ cells in the peripheral blood were evaluated according to previously published flow cytometry15. Briefly, for peripheral blood live-dead lymphocyte assay, blood was mixed and incubated with 10 μL Propidium iodide for 10 min at room temperature, then analyzed by flow cytometry. The analysis and gates were restricted to lymphocytes. For peripheral blood CD4+CD25+ cells, blood was mixed and incubated with 10 μL monoclonal FITC-labeled anti-human CD4 and PE-anti-CD25 for 30 min at room temperature. After that, the samples were fixed and analyzed by flow cytometry. The analysis and gates were restricted to live lymphocytes. All experiments were performed in triplicate and repeated three times.
Isolation and culture of peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated and cultured according to previously published methods15. In brief, lymphocyte Separation Medium (Flow Labs, McLean, VA) in combination with density gradient centrifugation was used to isolate PBMCs from heparinized peripheral blood. Cells were next cultured in RPMI1640 medium containing 10% fetal bovine serum (HyClone) and 20 μg/ml phytohaemagglutinin (Sigma) in an atmosphere of 5% CO2 at 37 °C. After twenty-four hours, cell-free supernatant was obtained and frozen at −70 °C until assayed.
Enzyme-linked immunosorbent assay (ELISA)
The cell-free supernatant IL-4, serum IL-2, IL-6, and endothelial cell markers were tested using ELISA kits (R&D Systems, USA) according to the manufacturer’s instruction. All experiments were performed in triplicate and repeated three times.
Western Blotting for ADAMTS13 antibody
The serum of producing recombinant ADAMTS13 antibody16 (100 ng/lane) was added to gel-loading buffer (50 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulphate, 0.1% bromophenol blue, 10% glycerol). Next, 7% sodium dodecyl sulphate polyacryl gel electrophoresis was performed in Tris-glycine buffer (pH 8.3). Migrated samples were transferred onto a pure nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). After blocking with skimmed milk, membranes were incubated with citrated plasma samples (diluted 1:100 in TBS, 5% skimmed milk, 0.05% Tween 20, pH 7.4) used as a possible source of anti-ADAMTS13 antibody (R&D Systems, USA). Bound antibodies were visualized using alkaline phosphatase-labeled anti-human immunoglobulin (diluted 1:2000) and an alkaline phosphatase conjugate substrate kit (AmershamBioscences, Uppsala, Sweden). The β-actin was probed as a control for equal protein loading. Experiments were repeated three times; each experiment was in triplicate.
Renal histopathology examination
Kidney injury was evaluated using histopathological methods according to previously published methods17. Briefly, kidney biopsy samples from 30 patients with primary MHTN related kidney injury were fixed (using 10% buffered formalin), dehydrated, embedded in paraffin, and examined in the slide via the hematoxylin and eosin staining, periodic acid-schiff, or immunofluorescence techniques. Renal histological and immunofluorescence results, including chronicity indices (CI), activity indices (AI), complement component C3, IgG, IgA, and IgM, were graded semi-quantitatively on a mild, moderate, and marked scale (also called 1+, 2+, and 3+ scale) as previously described18,19,20. In this study, CI consists of glomerular sclerosis, fibrous crescents, renal tubular atrophy and interstitial fibrosis; whereas AI was defined according to glomerular hypercellularity, leucocyte exudation, fibrinoid necrosis, cellular crescents, hyaline deposits and interstitial inflammation.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 16.0 (SPSS Inc., USA) and Graph Pad Prism 5.0 (Graph Pad Inc., USA). Data were checked for normality of distribution using the Kolmogorov-Smirnov test and expressed as mean ± standard deviation (SD) or median and interquartile range. Differences between the two groups were analyzed using t -test or Mann-Whitney U-test. The Spearman r test was used to analyze the relationships between different values. A p value < 0.05 was considered statistically significant.
Results
Flow cytometry analysis of peripheral blood live-dead lymphocytes
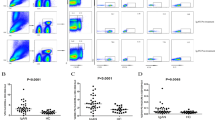
The results from flow cytometry analysis peripheral blood live-dead lymphocytes showed live lymphocytes accounted for 99% in both patients with MHTN related kidney injury and controls (CD3- FITC positive cells; PI negative cells); whereas the number of dead lymphocytes was less than 1% (Fig. 1). There were no differences between patients and controls in terms of the distribution of Live and Dead lymphocytes (all P > 0.05).
Flow cytometry analysis of CD4+CD25+ cells
We next analyzed the population of CD4+CD25+ cells in peripheral blood using the flow cytometry (Fig. 2). The results exhibited that the number of CD4+CD25+ cells was significantly decreased in patients with primary MHTN related kidney injury compared to controls (P < 0.05) (Table 1).
Clinical and histopathological findings in patients
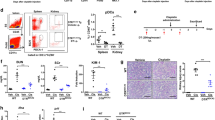
Table 2 summarizes clinical and histopathological findings in patients with MHTN related kidney injury. Serum IL-2, IL-6, supernatant IL-4, endothelial cell markers, ADAMTS13 antibody (Fig. 3), VCAM and vWF dramaticlly increased in patients compared to controls (*P < 0.05). Endothelial injury manifests with swelling and detachment of endothelial cells from the basement membrane, expansion of the subendothelial space, and newly formed basement membrane-like material. In arterioles, endothelial injury precedes myointimal swelling and proliferation, leading to vascular lumina narrowing or obliteration, secondary glomerular ischemia, which is characterized by glomerular tuft collapse, garland-like wrinkling and thickening of the capillary wall. Endothelial cell injury is likely the common determinant of a cascade of events that lead to irreversible renal failure (Figs 4 and 5)21.
ADAMTS13 antibody (positive %) accounted for 81% of the primary MHTN related kidney injury patients. Controls were negative. Lane M: Marker; lane 1: Controls; lane 2: Controls; lane 3: primary MHTN related kidney injury patients (Mild lesion); lane 4: primary MHTN related kidney injury patients (Moderate lesion); lane 5: primary MHTN related kidney injury patients(Marked lesion).
Number of CD4+CD25+ cells and clinical parameters
The association between the number of CD4+CD25+ cells and clinical parameters were examined in patients with primary MHTN related kidney injury (Table 3). The number of CD4+CD25+ cells was negatively correlated with the level of serum blood urea nitrogen (BUN) or uric acid, whereas positively correlated with eGFR (*P < 0.05). These results indicate that the number of CD4+CD25+ cells reflects renal function. In addition, the number of CD4+CD25+ cells were negatively correlated with the level of urine protein or supernatant IL-4 in patients (*P < 0.05).
Correlation between the number of CD4+CD25+ cells and histological classification of primary MHTN related kidney injury
The number of CD4+CD25+ cells in individualls with MHTN related kidney injury tended to decrease in parallel with increased the histological classification degrees of nephropathy, as determined by the classification19 (Fig. 5), however, the difference was not significant (Fig. 6).
Discussion
The pathogenesis of primary MHTN related kidney injury is unclear, although some factors such as direct pressure effects (forced dilatation), excessive vasoconstriction, volume depletion, endocrine/paracrine renin–angiotens, nitricoxide, natriuretic peptides, endothelins and oxidative stress might be related to the process22,23,24.
The CD4+CD25+ cells, a kind of important immunological modulatory cells, can reduce cytokine production and limit the development of a proinflammatory CD4+ Th2 phenotype25. The functionary deficiency or suppression of CD4+CD25+ cells might result in the inappropriate balance between allergen activation of CD4+CD25+ cells and effector Th2 cells26,27,28. This type of cells also plays a key role in immunomodulatory process following antigen inhalation29. Th1 responses (releasing IL-2, IFNγ, and TNF-β) are more prone to be regulated by CD4+CD25+ T cells than Th2 responses (releasing IL-4, IL-5, IL-6, IL-10, and IL-13)30. Our previous studies15,31,32 have shown that tonsillar CD4+CD25+ cells were significantly decreased in IgA nephropathy, multiple myeloma related renal impairment as well as thrombotic thrombocytopenic purpura (TTP) associated with systemic lupus erythematosus (SLE). Furthermore, we have found that tonsillar CD4+CD25+ cells from IgA nephropathy patients present reduced immunosuppressive activity in experimental IgA nephropathy rats33.
In the current study, CD4+CD25+ cells significantly decreased in patients with primary MHTN related kidney injury. Supernatant IL-4, serum IL-2 and IL-6, endothelial cell markers, ADAMTS13-antibody, VCAM, vWF, and urine protein and erythrocytes significantly elevated in those patients. The number of CD4+CD25+ cells was negatively related to serum BUN, uric acid and urinary protein, and supernatant IL-4; whereas positively related to eGFR. Furthermore, with increasing severity of histologically assessed nephropathy, the number of CD4+CD25+ cells tended to gradually decrease. However, it is unclear whether the decrease of this type cells is the cause or the consequence of the disease.
When patients with primary MHTN related kidney injury have decreased CD4+CD25+ cells, their lymphocytes would react significantly more strongly to antigens, leading to higher levels of cytokine (IL-2, IL-4, and IL-6) production. Excessive cytokine production in turn would enhance the production of antibodies against endothelial cell markers or ADAMTS13 antibody. Therefore, high levels of cytokine and antibodies are present in the blood, which directly damage vascular endothelium, as demonstrated by elevated levels of endothelial functional parameters vWF and VCAM. These changes lead to platelet abnormalities, complement activation or imbalance between blood coagulation and plasminogen, and thus contribute to the development of TMA, ischemia and damage34,35,36,37,38.
ADAMTS13 is a zinc-containing enzyme that cleaves large VWF multimers. Congenital or acquired deficiency of ADAMTS13 can increase their thrombogenicity. Reduced ADAMTS13 activity has been associated with TMA in MHTN39. We also detected elevated levels of anti-ADAMTS13 and anti-vWF antibodies in MHTN related kidney injury.
To sum up, CD4+CD25+ cells might play an important role in the pathogenesis of primary MHTN related kidney injury. The number of CD4+CD25+ cells tended to gradually decrease with increasing severity of histologically assessed nephropathy. Additional studies are necessary to demonstrate functional deficits or other biological properties of CD4+CD25+ cells in primary MHTN related kidney injury.
Additional Information
How to cite this article: Huang, H. et al. CD4+CD25+ T Cells in primary malignant hypertension related kidney injury. Sci. Rep. 6, 27659; doi: 10.1038/srep27659 (2016).
References
Lip, G. Y., Beevers, M. & Beevers, D. G. Do patients with de novo hypertension differ from patients with previously known hypertension when malignant phase hypertension occurs? Am J Hypertens. 13, 934–939 (2000).
Tara, A. et al. The role of the renin–angiotens in system in malignant vascular injury affecting the systemic and cerebral circulations. Progress in Biophysics & Molecular Biology. 84, 301–319 (2004).
Chike Nzerue et al. Malignant hypertension with thrombotic microangiopathy and persistent acute kidney injury (AKI). Clin Kidney. J. 7, 586–589 (2014).
Mervaala, E. et al. Cyclosporin a protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human rennin and angiotensinogen genes. Hypertension. 35, 360–366 (2000).
Muller, D. N. et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 35, 193–201 (2000).
Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Shevach, E. M., McHugh, R. S., Piccirillo, C. A. & Thornton, A. M. Control of T-cell activation by CD4+CD25+suppressor T cells. Immuno.l Rev. 182, 58–67 (2001).
Powrie, F., Read, S., Mottet, C., Uhlig, H. & Maloy, K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 252, 92–98; discussion 98–105, 106–114 (2003).
Shi, H.-Z. & Qin, X.-J. CD4+CD25+ regulatory T lymphocytes in allergy and asthma. Allergy. 60, 986 (2005).
Sakaguchi, S. et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 160, 1151–64 (1995).
Levings, M. K. et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor b, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196, 1335–46 (2002).
Khattri, R. et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Van der Merwe, W. & van der Merwe, V. Malignant hypertension: a preventable emergency. N Z Med. J. 126, 39–45 (2013).
Levey, A. S. & Stevens, L. A. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J .Kidney. Dis. 55, 622–627 (2010).
Huang, H. D. et al. CD4+CD25+ Treg Cells in thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus patients. Ren. Fail. 36, 1263–7 (2014).
De Cristofaro, R. et al. Role of chloride ions in modulation of the interaction between von Willebr and factor and ADAMTS-13. J. Biol Chem. 280, 23295–302 (2005).
Huang, H. et al. CD4+CD 25+ Treg cells and IgA nephropathy patients with tonsillectomy: a clinical and pathological study. Int. Urol. Nephrol. 46, 2361–9(2014).
Jiang, L. et al. Concise semiquantitative histological scoring system for immunoglobulin A nephropathy. Nephrology (Carlton). 14, 597–605 (2009).
Wu, L. H. et al. Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. KIDNEY INT. 83, 715–723 (2013).
Cohen, D. et al. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis & Rheumatism. 58, 2460–2469 (2008).
Ruggenenti, P. & Remuzzi, G. Malignant vascular disease of the kidney: nature of the lesions, mediators of disease progression, and the case for bilateral nephrectomy. Am J Kidney Dis. 27, 459–75 (1996).
Adam, A. & Raij, L. Nitricoxide–angiotensin II axis in renal and cardiovascular injury. J. Nephrol. 13, 211–220 (2000).
Fleming, S. Malignant Hypertension The role of the paracrine renin–angiotensinsystem. J.Pathol. 192, 135–139 (2000).
Ruiz-Ortega, M. et al. Role of the renin–angiotensin system in vascular diseases: expanding the field. Hypertension. 38, 1382–1387(2001).
Mottet, C., Uhlig, H. H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190, 995–1004 (1999).
Thornton, A. M. & Shevach, E. M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164, 183– 190 (2000).
Huang, H., Peng, Y., Liu, F. & Lei, H. Is IgA nephropathy induced by abnormalities of CD4+CD25+ Treg cells in the tonsils? Med. Hypotheses. 69, 410–413 (2007).
Akbari, O. et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8, 1024–1032 (2002).
McGuirk, P., McCann, C. & Mills, K. H. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells:a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195, 221–231 (2002).
Huang, H. D., Peng, Y. M., Liu, H., Yang, X. M. & Liu, F. Y. Decreased CD4+CD25+ cells and increased dimeric IgA-producing cells in tonsils in IgA nephropathy. J. Nephrol. 23, 202–209 (2010).
Hongdong, Huang et al. CD4+CD25+ cells in multiple myeloma related renal impairment. Sci Rep. 5, 16565 (2015).
Huang, H. D. et al. Tonsillar CD4+CD25+ Regulatory T Cells from IgA Nephropathy Patients Have Decreased Immunosuppressive Activity in Experimental IgA Nephropathy Rats. Am .J .Nephrol. 37, 472–480 (2013).
Boumpas, D. T. et al. Systemic lupus erythematosus: emerging concepts. Ann. Intern. Med. 123, 42–53 (1995).
D Cruz, D. R. et al. Antibodies to endothelial cells is sytemic lupus erythematosus: a potential marker for nephritis and vasculitis. Clin. Exp. Immunol. 85, 254–61 (1991).
Perry, G. J. et al. Antiendothelial cell antibodies in lupus: correlations with renal injury and circulating markers of endothelial damage. Q .J .Med. 86, 727–34 (1993).
Chu, R., Russell, N. H., Powell, R. J., Cater, D. R. & Harris, R. J. Abnormal fibrinolytic activity in systemic lupus erythematosus and possible mechanisms. Br. J. Rheumatol. 27, 436–9 (1988).
Awada, H. et al. Fibrinolysis abnormalities in systemic lupus erythematosus and their relation to vasculitis. J. Lab Clin Med. 111, 229–36 (1988).
Remuzzi, G. Is ADAMTS13 deficiency specific for thrombotic thrombocytopenic purpura? No. J. Thromb Haemost1. 1, 632–634 (2003).
Acknowledgements
This study was supported by the Foundation of Beijing Shijitan Hospital of Capital Medical University (Grant No. 2014-C11 and 2011-C06), The Research Foundation of Returned Overseas Chinese Scholars (Grant No. 2015-311), Hunan Provincial Natural Science Foundation (Grant No. 09jj6056), and the Foundation for Excellent Doctoral Education of Central South University (Grant No. 2340).
Author information
Authors and Affiliations
Contributions
H.D.H., Y.L., Y.M.L., X.D.L., Y.M.P., Z.H.L., X.J.W., M.J. and X.Y.W. were responsible for experimental design. H.D.H. supervised the study. X.D.L., Y.M.P., Z.H.L., X.Q.D., F.L.H. and Q.S.L. developed methodology. H.D.H., R.T., C.L.B., C.L., Y.M.L., X.D.L., Y.M.P., Z.H.L., X.J.W., X.Y.W. and M.J. carried out the experiments. X.J.W., M.J., X.Y.W., F.L.H., Q.S.L. and X.Q.D. performed data analysis. H.D.H., Y.M.L., X.D.L., Y.M.P., Z.H.L., X.J.W. and M.J. prepared the figures and tables. H.D.H., Y.L. and X.Q.D. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, H., Luo, Y., Liang, Y. et al. CD4+CD25+ T Cells in primary malignant hypertension related kidney injury. Sci Rep 6, 27659 (2016). https://doi.org/10.1038/srep27659
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27659
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.