Abstract
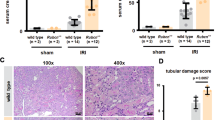
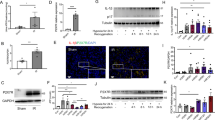
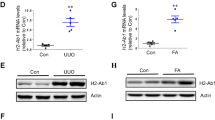
Acute kidney injury (AKI) is a common clinical complication associated with high mortality in patients. Immune cells and cytokines have recently been described to play essential roles in AKI pathogenesis. Plasmacytoid dendritic cells (pDCs) are a unique DC subset that specializes in type I interferon (IFN) production. Here, we showed that pDCs rapidly infiltrated the kidney in response to AKI and contributed to kidney damage by producing IFN-α. Deletion of pDCs using DTRBDCA2 transgenic (Tg) mice suppressed cisplatin-induced AKI, accompanied by marked reductions in proinflammatory cytokine production, immune cell infiltration and apoptosis in the kidney. In contrast, adoptive transfer of pDCs during AKI exacerbated kidney damage. We further identified IFN-α as the key factor that mediated the functions of pDCs during AKI, as IFN-α neutralization significantly attenuated kidney injury. Furthermore, IFN-α produced by pDCs directly induced the apoptosis of renal tubular epithelial cells (TECs) in vitro. In addition, our data demonstrated that apoptotic TECs induced the activation of pDCs, which was inhibited in the presence of an apoptosis inhibitor. Furthermore, similar deleterious effects of pDCs were observed in an ischemia reperfusion (IR)-induced AKI model. Clinically, increased expression of IFN-α in kidney biopsies was observed in kidney transplants with AKI. Taken together, the results of our study reveal that pDCs play a detrimental role in AKI via IFN-α.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yang, L. et al. Acute kidney injury in China: a cross-sectional survey. Lancet 386, 1465–71. (2015).
Okusa, M. D., Rosner, M. H., Kellum, J. A. & Ronco, C. Therapeutic targets of human AKI: harmonizing human and animal AKI. J. Am. Soc. Nephrol. 27, 44–48 (2016).
Lewington, A. J., Cerda, J. & Mehta, R. L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 84, 457–467 (2013).
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004).
Saitoh, S. I. et al. TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat. Commun. 8, 1592 (2017).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
Reizis, B., Bunin, A., Ghosh, H. S., Lewis, K. L. & Sisirak, V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev. Immunol. 29, 163–183 (2011).
Gregorio, J. et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 207, 2921–2930 (2010).
Arai, Y. et al. Plasmacytoid dendritic cell activation and IFN-alpha production are prominent features of murine autoimmune pancreatitis and human IgG4-related autoimmune pancreatitis. J. Immunol. 195, 3033–3044 (2015).
Tiberio, L. et al. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol. Immunol. 15, 346–52. (2018).
Lechner, J. et al. IFN-alpha induces barrier destabilization and apoptosis in renal proximal tubular epithelium. Am. J. Physiol. Cell Physiol. 294, C153–C160 (2008).
Winterberg, P. D. et al. Reactive oxygen species and IRF1 stimulate IFNalpha production by proximal tubules during ischemic AKI. Am. J. Physiol. Ren. Physiol. 305, F164–F172 (2013).
Coroneos, E., Petrusevska, G., Varghese, F. & Truong, L. D. Focal segmental glomerulosclerosis with acute renal failure associated with alpha-interferon therapy. Am. J. Kidney Dis. 28, 888–892 (1996).
Maazi, H. et al. Activated plasmacytoid dendritic cells regulate type 2 innate lymphoid cell-mediated airway hyperreactivity. J. Allergy Clin. Immunol. 141, 893–905.e6 (2018).
Castellaneta, A. et al. Plasmacytoid dendritic cell-derived IFN-alpha promotes murine liver ischemia/reperfusion injury by induction of hepatocyte IRF-1. Hepatology 60, 267–277 (2014).
Ruben, J. M. et al. Human plasmacytoid dendritic cells acquire phagocytic capacity by TLR9 ligation in the presence of soluble factors produced by renal epithelial cells. Kidney Int. 93, 355–64. (2018).
De Palma, G. et al. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int 79, 1228–1235 (2011).
Rabb, H. et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 27, 371–379 (2016).
Festa, B. P. et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 9, 161 (2018).
Deng, B. et al. The leukotriene B4-leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney Int 92, 89–100 (2017).
Jang, H. R. & Rabb, H. Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol. 11, 88–101 (2015).
Guo, C. et al. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 92, 1194–1205 (2017).
Watarai, H. et al. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc. Natl Acad. Sci. USA 105, 2993–2998 (2008).
Castellano, G. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 17, 72 (2015).
Yan, K. et al. Toll-like receptor 3 and RIG-I-like receptor activation induces innate antiviral responses in mouse ovarian granulosa cells. Mol. Cell Endocrinol. 372, 73–85 (2013).
Joshi, V. D. et al. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J. Interferon Cytokine Res. 26, 739–747 (2006).
Zuidwijk, K. et al. Increased influx of myeloid dendritic cells during acute rejection is associated with interstitial fibrosis and tubular atrophy and predicts poor outcome. Kidney Int. 81, 64–75 (2012).
Reich, B., Viehmann, S. F. & Kurts, C. Plasmacytoid dendritic cells: important players in human kidney allograft rejection. Kidney Int. 93, 301–303 (2018).
Famulski, K. S. et al. Molecular phenotypes of acute kidney injury in kidney transplants. J. Am. Soc. Nephrol. 23, 948–958 (2012).
Liu, J. & Dong, Z. Neutrophil extracellular traps in ischemic AKI: new way to kill. Kidney Int. 93, 303–305 (2018).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Kumar, S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 93, 27–40 (2018).
Tanaka, S., Inoue, T., Hossack, J. A. & Okusa, M. D. Nonpharmacological, biomechanical approaches to control inflammation in acute kidney injury. Nephron 137, 277–281 (2017).
Ramesh, G. & Reeves, W. B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110, 835–842 (2002).
Xia, C. Q. et al. Increased IFN-alpha-producing plasmacytoid dendritic cells (pDCs) in human Th1-mediated type 1 diabetes: pDCs augment Th1 responses through IFN-alpha production. J. Immunol. 193, 1024–1034 (2014).
Agalioti, T., Villablanca, E. J., Huber, S. & Gagliani, N. TH17cell plasticity: the role of dendritic cells and molecular mechanisms. J. Autoimmun. 87, 50–60 (2018).
Gonzalez-Navajas, J. M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Kretschmer, S. & Leekirsch, M. A. Type I interferon-mediated autoinflammation and autoimmunity. Curr. Opin. Immunol. 49, 96–102 (2017).
Nakazawa, D. et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J. Am. Soc. Nephrol. 28, 1753–1768 (2017).
Smith, N. et al. Natural amines inhibit activation of human plasmacytoid dendritic cells through CXCR4 engagement. Nat. Commun. 8, 14253 (2017).
Xu, Y. et al. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 26, 2647–2658 (2015).
Tullius, S. G. & Rabb, H. Improving the supply and quality of deceased-donor organs for transplantation. N. Engl. J. Med. 378, 1920–1929 (2018).
Barabas, K., Milner, R., Lurie, D. & Adin, C. Cisplatin: a review of toxicities and therapeutic applications. Vet. Comp. Oncol. 6, 1–18 (2010).
Rizza, P., Moretti, F. & Belardelli, F. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity. Autoimmunity 43, 204–209 (2010).
Launay-Vacher, V. et al. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 110, 1376–1384 (2007).
Aapro, M. & Launay-Vacher, V. Importance of monitoring renal function in patients with cancer. Cancer Treat. Rev. 38, 235–240 (2012).
Rascio, F. et al. A type I interferon signature characterizes chronic antibody-mediated rejection in kidney transplantation. J. Pathol. 237, 72–84 (2015).
Wei, D. et al. Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget 7, 17479–17491. (2016).
Swiecki, M., Gilfillan, S., Vermi, W., Wang, Y. & Colonna, M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33, 955–966 (2010).
Chan, A. J. et al. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am. J. Pathol. 184, 1411–1418 (2014).
Wendland, M. et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl Acad. Sci. USA 104, 6347–6352 (2007).
Ma, S. et al. Granulocyte and monocyte adsorptive apheresis ameliorates sepsis in rats. Intensive Care Med. Exp. 5, 18 (2017).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No: 81870462 and 81470990 to F. Ding; No: 91642112 to R.H.; and No: 31600715 to Y.L.L.); The Science and Technology Commission of Shanghai Municipality (No: 18140903300 to R.H.; No: 17441904200 and 19441909300 to F.D.); Shanghai Ninth People’s Hospital Clinical Research Program (No: JYLJ007 to F.D.); Shanghai Ninth People’s Hospital MDT Program (2017–1–019 to F.D.); Major Special Projects of the Ministry of Science and Technology (2018ZX10302207 to Y.L.L.); Development Project of Shanghai Peak Disciplines-Integrative Medicine (20180101 to Y.L.L.); Doctoral Innovation Fund Projects from Shanghai Jiao Tong University School of Medicine (BXJ201730 to B.D.); and Fundamental Research Program Funding of Ninth People's Hospital Affiliated with Shanghai Jiao Tong University School of Medicine (JYZZ082B to B.D.).
Author information
Authors and Affiliations
Contributions
F.D. and R.H. conceived and designed the research; B.D., Y.S., S.M., Q.C., W.J., B.L., T.Y. and P.H. performed the research; B.D., R.H. and F.D. analyzed the data; B.D. and Y.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Rights and permissions
About this article
Cite this article
Deng, B., Lin, Y., Chen, Y. et al. Plasmacytoid dendritic cells promote acute kidney injury by producing interferon-α. Cell Mol Immunol 18, 219–229 (2021). https://doi.org/10.1038/s41423-019-0343-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0343-9
Keywords
This article is cited by
-
A novel immune checkpoint score system for prognostic evaluation in pancreatic adenocarcinoma
BMC Gastroenterology (2023)
-
New insights into immune cell diversity in acute kidney injury
Cellular & Molecular Immunology (2023)
-
A comprehensive analysis of type 1 interferon gene signatures in systematic lupus erythematosus and prediction of the crucial susceptible factor for Sjögren syndrome
Clinical and Experimental Medicine (2023)
-
Cellular senescence in ischemia/reperfusion injury
Cell Death Discovery (2022)