Abstract
Lymphovascular invasion (LVI) is the primary and essential step in the systemic dissemination of cancer cells. The aim of our study was to assess the independent prognostic role of LVI for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. We retrospectively analyzed the clinical and pathological information of 206 patients diagnosed pT1 urothelial carcinoma with squamous differentiation. Of the 206 patients, LVI was detected in 57 (27.6%) patients. The 5 year cancer specific survival (CSS) rates were 87.2% in LVI (−) and 52.4% in LVI (+) (p < 0.001). According to univariate analysis, tumor multiplicity, tumor size, recurrence and LVI were the prognostic factors associated with CSS. Additionally, tumor size and LVI significantly influenced the CSS in multivariate analysis. TURBT had shorter median CSS than RC in recurred patients with LVI (+). Our study suggested that LVI is an important predictor for survival of pT1 urothelial carcinoma with squamous differentiation. LVI positive status and tumor size ≥3 cm led to a higher risk of death. RC should be routinely performed in recurred LVI (+) bladder cancer patients of pT1 urothelial carcinoma with squamous differentiation.
Similar content being viewed by others
Introduction
Squamous differentiation is well known to occur in the bladder urothelial carcinoma and represents the most common form of mixed differentiation1,2,3,4. Lymphovascular invasion (LVI) is the primary and essential step in the systemic dissemination of cancer cells5. Numerous studies have been conducted to elucidate significant prognostic factors for LVI in bladder cancer6,7,8,9,10,11,12. Within the spectrum of urothelial malignancy, the significance of LVI has been well characterized for bladder cancer, but not for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. The aim of our study was to assess the independent prognostic role of LVI for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. To the best of our knowledge, this is the first study focusing on clinical significance of LVI for pT1 urothelial carcinoma with squamous differentiation.
Patients and Methods
Patients
We retrospectively analyzed the clinical and pathological information of 206 patients who were diagnosed as pT1 urothelial carcinoma with squamous differentiation from 2003 to 2014 in our institution. Clinical data was collected by a retrospective review of the medical records. We excluded patients with a history of previous urothelial carcinoma and concomitant upper tract urothelial carcinoma. All the patients underwent transurethral resection of bladder tumor (TURBT) and intravesical chemotherapy. All recurrent patients underwent re-TURBT or radical cystectomy (RC) according to individual histological grade. All the patients were divided into 2 groups by LVI or not, and the recurred patients was divided into 2 groups by TURBT and RC. Cystoscopy has been suggested to be given during the postoperative follow-up according to the European and US guidelines13. The prognostic factors were assessed including age, gender, tumor grade, tumor multiplicity, tumor size, recurrence, and LVI. In order to eliminate interference of influencing factor of bladder tumor grading we redo the analysis in subgroup of low-grade and high-grade tumor with or without LVI. The prognostic implications of these factors on cancer specific survival (CSS) rates were analyzed. All demographic and pathological variables were queried. Variables were evaluated for inconsistencies and data integrity. The pathologic stage was based on the 2009 Union for International Cancer Control (UICC) TNM staging system4. Grade was based on the 2004 World Health Organization (WHO) grading system for non-invasive urothelial neoplasia2.
Pathology
All surgical specimens were submitted en bloc for pathological evaluation. Sectioning was performed on a case by case basis to provide adequate evaluation of grade and stage. Independent pathologic re-review of three representative slides from each patient was performed by two pathologists on all specimens to confirm reported pathologic findings and to confirm LVI status. The presence of intercellular bridges or keratinization was indicative of squamous differentiation. The presence of LVI in TURBT specimens was assessed using conventional hematoxylin and eosin (H&E) staining and immunohistochemical staining (IHC) markers against the lymphatic (D2-40) and vascular endothelium (CD 31)14,15. IHC assessment of LVI was performed on TURBT specimens of primary diagnosis. LVI was defined as the presence of the invasion of cancer cells into blood vessels or the lymphatic system or both and neoplastic cells within an endothelium-lined space.The criteria for diagnosing LVI did not change over the study period.
Statistical methods
Cancer specific survival was considered from the day of surgery to the day of bladder cancer specific death. The chi-squared test and Student’s t –test were used to evaluate the association between categorical and continuous variables, respectively. The Kaplan-Meier method was used to calculate overall survival trends, and differences were assessed using the log-rank statistic. Univariate and multivariate Cox regression models were used to analyze overall survival after operation. All reported P values were two-sided, and a P value of ≤0.05 was considered to indicate statistical significance. Statistical analysis was performed with SPSS software (Version 22).
Results
Clinical characteristics
Clinical and pathological characteristics are listed in Table 1. Mean patient age was 67.2 years old. Of the 206 patients, 170 were males and 36 were females with a 4.7:1 male-to-female ratio. Of the study population of 206 patients, LVI was detected in 57 (27.6%) patients. LVI positivity was not significantly associated with gender (P = 0.693), age (P = 0.749), tumor multiplicity (P = 0.169) or tumor size (P = 0.967). However, high grade tumors were more common in LVI (+) than in LVI (−) (71.9% versus 28.1%, P < 0.001). Recurrence was appeared in 65 (31.6%) patients of which LVI (+) was 25 (43.9%) and LVI (−) was 40 (26.8%) (P = 0.019). The patients of recurrence underwent TURBT or RC that was carried out by the surgeons were listed in Table 2.
Oncological outcome
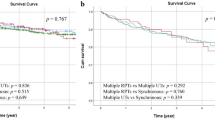
Fifty-one patients out of 206 (24.3%) died of cancer metastasis during the median follow up of 62.2 months (range 4 to 112 months). The overall 5 year CSS rate was 78.3%. Moreover, the 5 year CSS rates were 87.2% in LVI (−) and 52.4% in LVI (+) (P < 0.001, Fig. 1). In subgroup of low-grade, the 5 year CSS rates were 86.4% in LVI (−) and 48.2% in LVI (+) (P = 0.002, Fig. 2) and in subgroup of high-grade, the 5 year CSS rates were 88.6% in LVI (−) and 54.1% in LVI (+) (P < 0.001, Fig. 3). Disease recurred in 65 (31.6%) patients. However, recurrence was more common in LVI (+) than in LVI (−) (43.9% versus 26.8%, P = 0.019). We used Cox proportional hazard analysis for further analysis (Table 3). According to the results of univariate analysis, we found that tumor multiplicity (hazard ratio (HR) 1.778, 95% confidence interval (CI) 1.020–3.099, P = 0.042), tumor size (HR 1.936, 95% CI 1.103–3.399, P = 0.021), recurrence (HR 1.988, 95% CI 1.130–3.496, P = 0.017), and LVI (HR 3.774, 95% CI 2.167–6.571, P < 0.001) were the prognostic factors associated with CSS. However, in multivariate Cox proportional hazard analysis, only tumor size (HR 2.942, 95% CI 1.557–5.562, P = 0.001) and LVI (HR 4.806, 95% CI 2.550–9.055, P < 0.001) significantly influenced the CSS.
The Kaplan–Meier analysis was used to estimate CSS after recurrence stratified by TURBT versus RC in LVI (+) and LVI (−). Patients operated by TURBT had shorter median CSS duration than those operated by RC in LVI (+) (40.6 versus 56.4 months, P = 0.025, Fig. 4). However, no significant difference was observed between the two groups in LVI (−) (62.7 versus 63.6 months, P = 0.466, Fig. 5).
Pathology and immunohistochemistry
Squamous differentiation was observed and confirmed by pathologists in all 206 cases. The component of tumor was considered to be squamous when intercellular bridges and/or keratinization were evident (Fig. 6). The tumors showed strands or nests of infiltrating tumor cells with large and medium sized nuclei, often with nucleolus, and a not clearly separated amphophilic or eosinophilic cytoplasmic background. Stained sections in H&E were used to evaluate the presence of LVI (Fig. 7), IHC staining of CD31, CD24, and D34 was then performed. IHC stain in these cases were positive for CD31, CD24 and CD34 (Fig. 8).
Discussion
McDonald and Thompson reported the value of LVI as a criterion to assess the severity of urothelial bladder tumors for the first time16. Attention on the clinical significance of LVI in bladder cancer is growing, and a number of recent evidences have enhanced the significance of LVI for urothelial carcinoma of the bladder. Some papers indicated that LVI was an independent and significant prognostic factor for disease-specific survival17. Canter et al. analyzed the data from 356 patients treated with radical cystectomy by univariate analysis which found that the presence of LVI was a risk for overall, cancer-specific and recurrence-free survival (p < 0.0001)7. Cho et al., who conducted retrospective analyses of 118 patients reported that LVI, as an independent prognostic factor of progression and metastasis in pT1 bladder cancer, was significantly associated with disease recurrence18. The result was consistent with the study performed by Lopez and Angulo, in which multivariate analysis revealed that LVI was an independent prognostic factor in TURBT surgical specimens of T1 bladder cancer19.
However, there are short of data on the significance of LVI in patients of TURBT with urothelial carcinoma with squamous differentiation in bladder cancer, especially pT1 urothelial carcinoma of bladder. Our study showed further evidence suggesting that LVI was a pathological variable that might play an important role as a prognostic indicator in patients with pT1 urothelial carcinoma with squamous differentiation in bladder cancer. In our study, the presence of LVI was an independent prognostic factor related with disease survival (P < 0.001). In addition, there are hardly any data on the survival of the TURBT or RC in recurred patients in LVI for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. Our finding indicated that recurred patients operated by TURBT had shorter median CSS duration than those operated by RC in LVI (+) (P = 0.025). However, no significant difference was observed between the two groups in LVI (−) (P = 0.466). According to the results, we suggest surgeons should operate RC routinely in recurred patients for pT1 urothelial carcinoma with squamous differentiation with LVI (+) in bladder cancer.
Shariat et al. conducted a retrospective review of 4257 radical cystectomy specimens and stratified the patients by LVI status and pathological stage20. Akdogan et al. demonstrated that ureteral UC had a higher recurrence rate and poorer survival rate than renal pelvic UC21. Our series found that LVI (HR 3.774, 95% CI 2.167–6.571, P < 0.001) was the independent prognostic factors associated with CSS in both univariate analysis and multivariate analyses. In addition, tumor multiplicity (HR 1.778, 95% CI 1.020–3.099, P = 0.042), tumor size (HR 1.936, 95% CI 1.103–3.399, P = 0.021), and recurrence (HR 1.988, 95% CI 1.130–3.496, P = 0.017) were the prognostic factors associated with CSS, too. However, in multivariate Cox proportional hazard analysis, only LVI (HR 4.806, 95% CI 2.550–9.055, P < 0.001) and tumor size (HR 2.942, 95% CI 1.557–5.562, P = 0.001) significantly influenced the CSS.
The presence of LVI in patients with newly diagnosed T1 urothelial carcinoma in bladder cancer is associated with decreased recurrence-free survival18. T1 urothelial carcinoma in bladder cancer accounts for about 30% of non-muscle-invasive bladder tumors, with varying degrees of aggressiveness and progression rates of up to 50%22,23. The lamina propria lying just beneath the epithelial lining is rich in lymphatic and blood vessels that allows for early lymphatic and hematogenous tumor spread24. In our study, the tumors showed strands or nests of infiltrating tumor cells with large and medium sized nuclei, often with nucleolus, and a not clearly separated amphophilic or eosinophilic cytoplasmic background. Stained sections in H&E were used to evaluate the presence of LVI. IHC stain in these cases were positive for CD31, CD24 and CD34.
Conclusions
LVI is an important predictor for survival for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. LVI positive status and tumor size ≥3 cm led to a higher risk of death which led to a higher mortality rate. Surgeons should operate RC routinely in recurred patients for pT1 urothelial carcinoma with squamous differentiation with LVI (+) in bladder cancer.
Additional Information
How to cite this article: Li, G. et al. Poor prognostic value of lymphovascular invasion for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. Sci. Rep. 6, 27586; doi: 10.1038/srep27586 (2016).
References
Lopez-Beltran, A. et al. Squamous differentiation in primary urothelial carcinoma of the urinary tract as seen by MAC387 immunohistochemistry. J Clin Pathol 60, 332–335, doi: 10.1136/jcp.2006.038802 (2007).
Lopez-Beltran, A. & Cheng, L. Histologic variants of urothelial carcinoma: differential diagnosis and clinical implications. Hum Pathol 37, 1371–1388, doi: 10.1016/j.humpath.2006.05.009 (2006).
Emerson, R. E. & Cheng, L. Immunohistochemical markers in the evaluation of tumors of the urinary bladder: a review. Anal Quant Cytol Histol 27, 301–316 (2005).
Antunes, A. A. et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol 33, 339–345, discussion 346 (2007).
Sundar, S. S. & Ganesan, T. S. Role of lymphangiogenesis in cancer. J Clin Oncol 25, 4298–4307, doi: 10.1200/JCO.2006.07.1092 (2007).
Horikawa, Y. et al. Lymphatic invasion is a prognostic factor for bladder cancer treated with radical cystectomy. Int J Clin Oncol 12, 131–136, doi: 10.1007/s10147-006-0637-7 (2007).
Canter, D. et al. The presence of lymphovascular invasion in radical cystectomy specimens from patients with urothelial carcinoma portends a poor clinical prognosis. BJU Int 102, 952–957, doi: 10.1111/j.1464-410X.2008.07732.x (2008).
Lotan, Y. et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol 23, 6533–6539, doi: 10.1200/JCO.2005.05.516 (2005).
Kunju, L. P. et al. Lymphovascular invasion of urothelial cancer in matched transurethral bladder tumor resection and radical cystectomy specimens. J Urol 180, 1928–1932; discussion 1932, doi: 10.1016/j.juro.2008.07.056 (2008).
Reuter, V. E. Lymphovascular invasion as an independent predictor of recurrence and survival in node-negative bladder cancer remains to be proven. J Clin Oncol 23, 6450–6451, doi: 10.1200/JCO.2005.05.033 (2005).
Quek, M. L. et al. Prognostic significance of lymphovascular invasion of bladder cancer treated with radical cystectomy. J Urol 174, 103–106, doi: 10.1097/01.ju.0000163267.93769.d8 (2005).
Algaba, F. Lymphovascular invasion as a prognostic tool for advanced bladder cancer. Curr Opin Urol 16, 367–371, doi: 10.1097/01.mou.0000240311.08701.55 (2006).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64, 639–653, doi: 10.1016/j.eururo.2013.06.003 (2013).
O’Donnell, R. K., Feldman, M., Mick, R. & Muschel, R. J. Immunohistochemical method identifies lymphovascular invasion in a majority of oral squamous cell carcinomas and discriminates between blood and lymphatic vessel invasion. J Histochem Cytochem 56, 803–810, doi: 10.1369/jhc.2008.950790 (2008).
Manoharan, M. et al. Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World J Urol 28, 233–237, doi: 10.1007/s00345-009-0448-3 (2010).
Mc, D. J. & Thompson, G. J. Carcinoma of the urinary bladder; a pathologic study with special reference to invasiveness and vascular invasion. J Urol 60, 435–445 (1948).
Palmieri, F. et al. Prognostic value of lymphovascular invasion in bladder cancer in patients treated with radical cystectomy. Anticancer Res 30, 2973–2976 (2010).
Cho, K. S. et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol 182, 2625–2630, doi: 10.1016/j.juro.2009.08.083 (2009).
Lopez, J. I. & Angulo, J. C. The prognostic significance of vascular invasion in stage T1 bladder cancer. Histopathology 27, 27–33 (1995).
Shariat, S. F. et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int 105, 1402–1412, doi: 10.1111/j.1464-410X.2010.09217.x (2010).
Akdogan, B. et al. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol 176, 48–52, doi: 10.1016/S0022-5347(06)00511-8 (2006).
Shahin, O., Thalmann, G. N., Rentsch, C., Mazzucchelli, L. & Studer, U. E. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J Urol 169, 96–100, discussion 100, doi: 10.1097/01.ju.0000035543.69161.58 (2003).
Peyromaure, M. et al. Intravesical bacillus Calmette-Guerin therapy for stage T1 grade 3 transitional cell carcinoma of the bladder: recurrence, progression and survival in a study of 57 patients. J Urol 169, 2110–2112, doi: 10.1097/01.ju.0000066840.42991.4a (2003).
Stein, J. P. & Penson, D. F. Invasive T1 bladder cancer: indications and rationale for radical cystectomy. BJU Int 102, 270–275, doi: 10.1111/j.1464-410X.2008.07743.x (2008).
Acknowledgements
This study was supported by Tianjin Research Program of Application Foundation and Advanced Technology (number, 14CYBJC29800) and Science and technology innovation fund projects of Tianjin Institute of Urology (number, MNYB201503).
Author information
Authors and Affiliations
Contributions
G.L. designed the study, performed the data collection and revised the manuscript. H.S. wrote the manuscript, analyzed the data and discussed the results. J.W. critically reviewed revising the manuscript. Y.B. contributed the figures. Y.N. designed the study. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, G., Song, H., Wang, J. et al. Poor prognostic value of lymphovascular invasion for pT1 urothelial carcinoma with squamous differentiation in bladder cancer. Sci Rep 6, 27586 (2016). https://doi.org/10.1038/srep27586
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27586
This article is cited by
-
Quantitative evaluation of iodine and fat using dual-energy CT for assessments of the tumor aggressiveness in lung cancer
Egyptian Journal of Radiology and Nuclear Medicine (2022)
-
Risk stratification of postoperative recurrence in hypopharyngeal squamous-cell carcinoma patients with nodal metastasis
Journal of Cancer Research and Clinical Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.