Abstract
We report on damage to DNA in an aqueous medium induced by ultrashort pulses of intense laser light of 800 nm wavelength. Focusing of such pulses, using lenses of various focal lengths, induces plasma formation within the aqueous medium. Such plasma can have a spatial extent that is far in excess of the Rayleigh range. In the case of water, the resulting ionization and dissociation gives rise to in situ generation of low-energy electrons and OH-radicals. Interactions of these with plasmid DNA produce nicks in the DNA backbone: single strand breaks (SSBs) are induced as are, at higher laser intensities, double strand breaks (DSBs). Under physiological conditions, the latter are not readily amenable to repair. Systematic quantification of SSBs and DSBs at different values of incident laser energy and under different external focusing conditions reveals that damage occurs in two distinct regimes. Numerical aperture is the experimental handle that delineates the two regimes, permitting simple optical control over the extent of DNA damage.
Similar content being viewed by others
Introduction
Due to ready availability of ultrashort pulsed laser sources, investigations of how such pulses propagate through transparent media have gained considerable contemporary research interest. The drivers for these investigations involve both the basic understanding of the underlying physics1,2,3 as well as the tantalizing prospects of a plethora of applications like remote sensing4,5 and remote control6 of processes that occur in the earth’s atmosphere, broadband spectroscopy7,8, modification of materials9,10,11,12 and bond-selective chemistry13. Interestingly, the potential for applications has, in recent years, begun to infringe upon the domain of the life sciences: experiments have been reported in which ultrashort, intense laser pulses have probed the possibility of non-invasively monitoring stress-related proteins in human saliva14; such pulses have also become of utility in medical applications like dental and eye surgery15. A break in a strand of DNA constitutes damage that can occur either naturally or via artificial means. Filamentation-induced damage has recently been demonstrated in biomolecules such as DNA kept under physiological conditions16,17,18. It has been suggested that detrimental dose distributions within tissue that are irradiated by gamma radiation - one of the major difficulties in radiotherapy - might be avoided by use of femtosecond laser induced filamentation18. This is due to ultrashort laser pulses, particularly in the infrared region, being spatially confined to volumes (~125 μm3) that are very much smaller than what is possible to attain using contemporary clinical radiation sources. There is some evidence that 800 nm laser pulse induced filamentation can yield essentially the same radiation dosage in the radiolysis of water as that obtained using very energetic γ-radiation19.
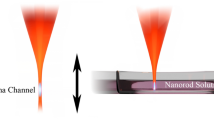
Filamentation and supercontinuum generation are spatial and temporal manifestations, respectively, of how ultrafast pulses of intense light propagate through matter. Supercontinuum generation is a consequence of self-phase modulation (SPM)20,21 in tandem with a complex interplay of a gamut of processes, such as ionization-enhanced SPM22, four-wave parametric processes, self-steepening, group velocity dispersion and shock waves23,24,25,26,27,28,29. At incident power levels in excess of the critical power for self-focusing (typically ~4 MW for intense 800 nm light in water and about three orders of magnitude larger in air) the optical Kerr effect causes the beam to self-focus. Upon reaching a small enough volume, the peak intensity of the self-focused beam can attain values that are high enough (~1012 W cm−2) to induce ionization of the medium, thereby creating electrons whose negative index contribution leads to defocusing of the beam. Along with diffraction, the dynamic balance that is set up leads to a series of focusing-defocusing cycles that enables the incident laser pulse to propagate to distances very much larger than the Rayleigh range, leaving behind a plasma channel with typical densities as large as ~1018 cm−3 being attained. Water molecules are ionized and dissociated within such a plasma channel, giving rise to low-energy electrons and OH-radicals16,17. These in-situ particles are utilized by us to probe electron- and radical-induced damage to DNA in an aqueous environment with a view to attaining an optical method to control the extent of damage that is induced, as described in the following.
In practice, filamentation, or formation of plasma channels, is achieved by externally focusing the incident laser beam. Competition between optical breakdown and filamentation in water was first investigated by Chin and coworkers30 in experiments that established the possibility of utilizing external focusing conditions to yield filamentation without breakdown, breakdown without filamentation and filamentation with breakdown. Values of NA used in these studies spanned the range from 0.034 to 0.231. Theoretical simulations whose results are in accord with these experimental findings have subsequently been reported31. The effect of geometrical focusing on parameters like filament length within condensed media, such as a BaF2 crystal32 and plasma density in air33, has been studied. Very recently, a comprehensive numerical and experimental study was carried out on how filamentation in air can also be altered by the numerical aperture of the external optics34. Values of NA used in these studies spanned the range from 0.00085 to 0.011. Two distinct regimes were identified which depend on NA. For high values of NA, external (geometrical) focusing as well as plasma effects govern the filamentation dynamics. On the other hand, at low values of NA, it is the Kerr nonlinearity - that underpins the self-focusing-defocusing cycle referred to above - that dominates filamentation dynamics. The transition value of NA delineates linear and nonlinear focusing regimes, with different physical mechanisms dominating the dynamics in the two regimes. We explore here the possibility of utilizing external optics to affect the type of damage induced in DNA (SSBs or DSBs) and its extent. Our experiments are carried out in water and conducted at an order of magnitude higher values of NA than those used earlier. Values of NA used in our experiments spanned the range from 0.015 to 0.09. As has been shown earlier16,17, simple considerations of nonlinear absorption of incident laser light fail to properly account for the dynamics that drive plasma-mediated DNA damage. The results that we present offer clear indications that the extent and nature of DNA damage can be controlled optically simply by altering the numerical aperture of the external optics. We believe that our results provide a ready handle for optimizing this laser-based ionizing source for biological and biomedical applications.
Results and Discussion
We exposed plasmid DNA (pBR322) to plasma channels created in water (in which the plasmids were suspended). The extent of resulting damage was quantified using gel electrophoresis. As has already been reported by us16,17, formation of bubbles (including microbubbles) accompanies formation of the plasma channel over the range of irradiance values achieved in our experiments. In the present experiments, bubbles were clearly visible over the range of incident laser energies we used, for all NA values. However, at the lowest laser energies, bubbles were not always clearly visible: microbubbles were formed which had to be imaged on a CCD camera using a microscope objective. The time evolution of bubble diameter as a function of irradiation conditions follows complex dynamics35 and results pertaining to the present experimental conditions will be presented elsewhere. Under normal conditions, for a given preparation of plasmid DNA, around 80–99% of DNA are expected to be in their usual supercoiled state. A schematic depiction of such supercoiled geometry is shown in Fig. 1a. Between 1% to 20% of the population is usually found to possess a relaxed, open-circular geometry which results from single stand breaks (SSBs) that may be induced by a host of extraneous events (including handling of DNA in the course of preparation, interactions with cosmic rays, ultraviolet radiation, oxidizing agents and such like). Our results (Fig. 1b) show that upon irradiation by 800 nm pulses (for up to 180 s) the resulting conformational changes are dramatic, with more than 80% becoming relaxed when the shortest focal length lens is used (generating the highest intensity); more than 50% become relaxed even at the lowest intensity that is obtained when we used the longest focal length lens (30 cm). Upon irradiation, less than 5% of DNA plasmids are seen to maintain their initial supercoiled structure. As many as 5% become linear. Our observations of the supercoiled → relaxed transformation are in agreement with earlier results obtained in near-IR experiments conducted at considerably lower intensity values16; tighter focusing and higher incident energy also permitted the occurrence of DSBs.
(a) Gel images obtained after pBR322 plasmid was irradiated with laser light using a 10 cm focal length lens. The negative and positive signs above the image panel indicate, respectively, no laser exposure and laser exposure for 180 seconds. Also shown are schematic depictions of single strand breaks (SSBs) and double strand breaks (DSBs) induced upon laser irradiation. Linear DNA results from DSBs. (b) Dependence of the percentage of DNA in supercoiled, relaxed and linear states on the focal length of the external lens. The bars marked “Control” pertain to DNA prior to irradiation. (c) Dependence of the percentage of DNA in supercoiled, relaxed and linear states for focal lengths of 8.5 cm to 12.5 cm. In each case irradiation was for 180 seconds using 820 nm laser light with the laser energy kept fixed (230 μJ) for different lenses.
We observed that, in the case of 5 cm lenses, only SSBs are induced at incident energy values of 2 μJ. At higher energy values, DSBs also manifest themselves in the form of linear DNA, as seen in Fig. 1a obtained after 180 s exposure at an energy of 230 μJ. The linearization of DNA is a clear-cut signature of the occurrence of DSBs wherein two complementary strands of the DNA are simultaneously damaged. In the cellular context, this is the most lethal form of DNA damage, one that might lead to cell death or cancer if left unrepaired36. Within cells DSBs can occur due to many factors, such as oxidative damage by free radicals, ionizing radiation like X-rays37 and UV radiation38. DSBs generally constitute a small percent of the total damage39 but they are, of course, very pernicious. DSBs were, until recently, thought to be caused exclusively by high-energy radiation but recent work16,17 has shown that both SSBs and DSBs are induced within the laser-induced plasma channels formed in water. Thermal effects also induce SSBs, more so when longer laser wavelengths are employed17; however, they have no role to play in inducing DSBs.
Plasma formation upon propagation through water of intense (~100 TW cm−2) femtosecond laser pulses has been theoretically modeled40 by treating water as an amorphous semiconductor whose band gap is generally taken to be 6.5 eV41 although recent work has offered indications that the value is closer to 8 eV42. Ionization of water molecules occurs via both multiphoton absorption as well as tunneling; the ionized electrons are further accelerated by the optical field - by inverse Bremsstrahlung - before hydration sets in on relatively long time scales (in excess of a few picoseconds). In case of optical breakdown in water electron densities of 1018–1020 cm−3 have been deduced40. These low-energy (≤5 eV) electrons readily take part in dissociative attachment collisions with H2O: multiple transient negative ion states are formed within DNA which rapidly decay into damaged structures19,43,44,45. In contrast, high energy radiation induces such strand breakages mostly as a consequence of the sugar-phosphate backbone being ionized. Thus, femtosecond laser-induced breakdown may be regarded - in a loose sense - as resembling the effects of high energy ionizing radiation, such as γ-rays.
In our experiments on aqueous DNA, the key initiator of the damage-inducing dynamics is the strong optical field that is the precursor to excitation, ionization and dissociation of H2O, yielding species like electronically excited H2O*, H2O+, OH, OH* and low-energy ionized electrons46. Solvated electrons are long-lived enough to participate in the dynamics we describe here; their lifetime values are estimated to range from 300 ns47 to ~500 ps48. As discussed later, collisions between electrons and H2O can yield electronically excited H2O*. In turn, collisions between H2O* and H2O+ give rise to the formation of OH radicals, H2O* + H2O+ → OH + H3O+. Slow electrons, of specific energy, can also attach to H2O via a resonant process known as dissociative attachment, e + H2O → H2O− → OH + H−. For instance, 7 eV electrons lead to formation of an H2O− state that survives for a few hundred attoseconds49 before dissociating. It is the slow electrons and OH-radicals that are generated, in situ, in strong-field interactions with H2O that, in turn, induce transformation of DNA that we seek to explore.
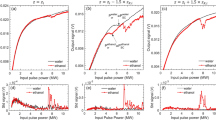
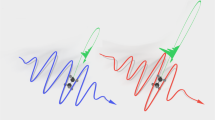
Is it possible to exert experimental control over the extent of damage that is induced by ultrashort laser irradiation? We explore this possibility by quantifying the effect of external focusing of the laser beam that is incident on the water+DNA sample. Typical results are shown in Fig. 1b,c in which the percentage of supercoiled, relaxed and linear DNA is monitored as a function of the focal length of the lens used, keeping the incident laser energy at a fixed value (230 μJ). As the focal length is varied from 5 cm to 30 cm, the numerical aperture changes from 0.09 to 0.015. Perhaps more significantly from an experimental viewpoint, the confocal volume within which laser-DNA interactions take place changes from a compact 150 μm3 for f = 5 cm to more than 32000 μm3 for f = 30 cm. These numbers are computed without taking into account the fact that plasma formation, especially at high NA values, will make the effective confocal volume larger30, although the extent of such enhancement is difficult to quantify experimentally. The dependence of both parameters on focal length is shown in Fig. 2. The upper panel depicts, in cartoon form, two distinct regimes. At high NA values, where tight focusing is obtained using short focal length lenses, the interaction volume (confocal volume) is very small. On the other hand, for low NA values that are obtained when longer focal length lenses are used, the interaction (confocal) volume is larger: it takes the form of an extended plasma channel. For purposes of later discussion, we denote the high NA regime as Regime I and the low NA regime as Regime II. The observation that the percentage of relaxed and linear DNA does not change monotonically either with confocal volume or with incident energy indicates that an interplay of both factors determines the overall dynamics that cause strand breakages.
We note that the maximum energy to which the ionized electrons are accelerated is >5 eV at an intensity of 100 TW cm−2; it may be as high as a few hundred eV at 10 PW cm−2. Electron attachment is generally a resonant process but its overall cross section falls off very rapidly as electron energy increases. Thus, we anticipate that electrons play little or no role in inducing strand breakages in the high intensity regime that is accessed in our experiments. At incident energy of 2 μJ we observe SSBs while at 230 μJ we also observe DSBs in the case of the f = 5 cm lens. On increasing the focal length we observe an increase in the percentage of DSBs for the f = 10 cm lens but a reduction in DSB percentage for f = 15 cm. Further increase in focal length results in increase in DSB percentages.
It is clear from our results (Fig. 1b,c) that there are two regimes that play a role in our experiments: the two regimes are delineated by NA. The two regimes are further exemplified in Fig. 3 where we discuss the biologically important result pertaining to linearization of initially supercoiled DNA by seeking an answer to the important question: Is it the electrons or the OH-radicals formed upon strong-field interactions with H2O that induce the conformational changes (supercoiled → relaxed, supercoiled → linear and relaxed → linear) that we observe under different external focusing conditions? To probe this question we added electron- and OH-scavengers to the DNA+ water sample; sodium acetate is an OH-radical scavenger while 5-bromouracil is predominantly an electron scavenger. We investigated how DNA damage is affected in the presence of sodium acetate (over the concentration range 0–200 mM) and 5-bromouracil (over the concentration range 2–65 mM). On the basis of such concentration dependent measurements, we deduced that both electrons and OH radicals induce damage in DNA but that the latter is four times more pernicious than the former16. The relative invariance of percentages observed in relaxed DNA indicates clearly that electrons play little or no role in strand breakages that we observe in these experiments (inset of Fig. 3). As noted above, this is consistent with the electron energies under our experimental conditions being too high for attachment processes to occur with reasonable efficiency. In this context we note that higher-energy electrons (~7 eV) can, indeed, contribute to formation of H2O− states49 but their ultrashort lifetime, of a few hundred attoseconds, preclude a significant role in inducing DNA damage. On the other hand, the results depicted in Fig. 3 show that the presence of the OH-scavenger strongly affects the percentages of relaxed species. Under our experimental conditions - high intensity irradiation by 800 nm light - it may be the OH-radicals that are overwhelmingly responsible for DNA strand breakages.
Multiphoton excitation of DNA, which exhibits maximum linear absorption around 260 nm wavelength, might be expected to cause a variety of lesions, including DSBs50,51. However, our earlier experiments conducted at 1350 nm and 2200 nm wavelength17 have established that the extent of damage is not wavelength dependent and, consequently, multiphoton effects are unlikely to play a direct role in the strand breakage dynamics. Strand breakages are most likely induced by indirect effects that occur as the strong optical field interacts with H2O. Interactions of high energy x-rays and γ-rays with water give rise to OH formation which, in reactions with DNA, accounts for the majority of radiation damage to cellular systems52. Despite the reactions of OH radicals with the DNA (composed of a series of smaller molecules called nucleotides, with each nucleotide made up of nitrogenous base, sugar molecule called deoxyribose and a phosphate group attached to the sugar molecule) have been investigated both experimentally and theoretically (see53,54 and references therein). However, the mechanism of OH-induced DNA damage are yet to be elucidated. Experimental evidence suggests that hydrogen abstraction mainly leads to damage in the form of SSBs which, as already noted, are amenable to repair. The occurence of DSBs, on the other hand, seems to require interactions involving electronically excited states of OH17 which are produced when H2O is electronically excited and then predissociates into OH*. For energies in excess of ~9 eV, direct dissociation of H2O* is adiabatically correlated to OH fragments in the excited A2∑+ state. In order to explore the efficacy of excited OH to induce DSBs, we made measurements at various values of incident laser energy. As is seen from the results shown in Fig. 4, measurable percentages of linear DNA are obtained only at laser energy in excess of 50 μJ. At lower energy levels it is likely that the energy gained by ionized electrons is insufficient to electronically excite H2O, precluding formation of excited OH*. The mechanism involved in OH*+DNA interactions leading to DSBs remains to be elucidated, mainly because of the currently intractable nature of the problem of understanding OH reactivity in an aqueous medium. The root of the problem arises due to the dynamics being dependent on the arrangement and conformations of all neighboring H2O molecules. It has been computationally demonstrated that by simply changing the water conformation the potential barrier for OH-induced hydrogen abstraction from a methane molecule alters by more than a factor of two55. Symptomatic of the difficulties of modeling is the computational demonstration in the case of guanine53 of the OH-induced hydrogen abstraction energy from the N1H or NH2 site increasing from its zero gas-phase value (indicating no barrier) to as much as 7–10 kcal/mol when guanine is solvated by only a dozen water molecules.
It is interesting to note in the context of our present work that OH-induced strand breakages are strongly dependent on external focusing conditions. This is clearly brought to the fore in results depicted in Fig. 5 where the percentage of linear DNA is plotted as a function of the focal length of the external focusing optics. These measurements were made at an incident energy of 230 μJ. We observe DSBs at NA values of around 0.09, obtained with a 5 cm lens. Increasing the focal length we observe an increase in the percentage of DSBs for a 10 cm lens (where the NA is 0.045). This case corresponds to the situation wherein both optical breakdown and filamentation are operative30. On reducing the NA, or increasing the focal length of the external lens, we observe a slight reduction in the DSB percentage which, upon further decrease in NA value (further increase in focal length) again produces an increase in DSB percentage. The functional dependence shown in Fig. 5 clearly allows demarcation of two distinct regimes: regimes I and II.
Variation in the percentage of linear DNA as a function of the focal length of the external lens.
These measurements were made at an incident laser energy of 0.23 mJ. The value of numerical aperture (NA) for the 5 cm lens is 0.09; the corresponding value for the 30 cm lens is 0.015. As shown in Fig. 2, high NA values are denoted as Regime I while low NA values designate Regime II.
Experiments conducted using a 5 cm and 8.5 cm lenses show that geometric focusing plays a key role in restricting the region where low energy electrons and OH radicals are generated. For the 10 cm lens, we are operating close to the transition region where even though the effect of geometric focusing may be somewhat reduced, the Kerr focusing provides an extended region (Fig. 2) for generation of low energy electrons and OH radicals. At even higher focal lengths (12.5 cm and beyond) the Kerr focusing plays the dominant role; the external focusing appears to exert correspondingly less influence. In this regime electron energies can be large enough to induce formation of OH radicals in rotationally hot states. Although the intensities within the plasma channel may be clamped the accompanying spatial extension of the plasma channel in the Kerr focusing regime (Regime II) leads to a larger propensity for DSBs to occur. This is clearly reflected in our data pertaining to the occurrence of SSBs and DSBs as a function of the focal length of the external lens.
Summary
In summary, we conducted experiments to probe damage to aqueous DNA upon interactions with low-energy electrons and OH-radicals produced in plasma channels formed in water. Our measurements have used DNA damage as a readout. Our results provide evidence for single and double strand breakages occurring in two distinct regimes: low NA and high NA. Our method relies on a novel use of strong-field interactions with water wherein electrons and free radicals are generated in situ upon multiphoton and tunneling ionization and dissociation of H2O. The low-energy electrons and OH radicals interact with DNA plasmids under physiological conditions, producing nicks. We quantify the damage caused by using electron and OH-scavengers. Our experiments offer indications that OH-radicals are mainly responsible for formation of DSBs, with a prominent role being played by electronically excited OH* radicals that are produced upon pre-dissociation of electronically excited H2O* states. Such electronically excited states of H2O are, of course, themselves formed from interactions involving electrons in the plasma channel that is induced in water upon intense laser irradiation. We have carried out systematic quantification of SSBs and DSBs at different values of incident laser intensity (keeping the focal length of the external lens constant) as well as under different external focusing conditions. We have demonstrated the feasibility of employing a simple optical method to vary the extent of damage in DNA. Our findings have implications beyond studies of damage to DNA per se. Our experimental technique of generating, in situ, slow electrons and radicals within aqueous media has important implications in different scenarios where the effects of non-ionizing radiation need to be probed under physiologically relevant conditions.
Methods
Ultrashort pulses of 800 nm laser light are generated from an Ti:sapphire amplifier operating at 1 kHz repetition rate that has been described in several recent reports17,46. Using spectral shear interferometry the incident laser pulse duration was measured to be 40 fs. The incident beam had a M2 value of 1.3. and the beam diameter was 9 mm. Different lenses of focal lengths in the range 5 to 30 cm were used to carry out irradiation of our DNA sample for a period of 180 seconds.
Our DNA (pBR322), obtained from a commercial source (Merck-Millipore, India). The samples were dispensed into convenient volumes and stored at −20 C. The concentration of DNA was spectrophotometrically determined and we standardized the amount of DNA to yield maximum nicking, establishing a working range of 2–6 × 1011 molecules in 300 μℓ sample volume. We found that the lower end of this range yielded the best percentage of relaxed species following laser irradiation for 180 s. The concentration of our plasmid DNA was measured to be in the range 1.9–3.8 × 1011 cm−3, corresponding to concentrations of 0.9–1.8 μg per 300 μℓ, out of which ~3 × 108 plasmids were expected to be within the plasma channel (the confocal volume) - constituting 0.03% of plasmids. Related work16,17 carried out in our laboratory has established that strong thermal gradients are set up as our intense laser beam propagates through water +DNA, giving rise to convective flow. Thus, DNA molecules within the confocal volume are constantly replenished.
After irradiation, DNA fragments were separated using gel electrophoresis. Post-separation, the gel was stained with a DNA binding fluorescent dye, ethidium bromide, which enabled us to image and carry out quantification using a BIORAD Gel Documentation system in conjunction with standard gel-analysis software (ImageJ). We made use of commercially available DNA ladders containing linear fragments of known length to identify the DNA fragments.
Additional Information
How to cite this article: Dharmadhikari, J. A. et al. Optical control of filamentation-induced damage to DNA by intense, ultrashort, near-infrared laser pulses. Sci. Rep. 6, 27515; doi: 10.1038/srep27515 (2016).
References
Couairon, A. & Mysyrowicz, A. Femtosecond filamentation in transparent media. Phys. Rep. 441, 47–189 (2007).
Bergé, L., Skupin, S., Nuter, R., Kasparian, J. & Wolf, J.-P. Ultrashort filaments of light in weakly ionized, optically transparent media. Rep. Prog. Phys. 70, 1633–1713 (2007).
Couairon, A., Chakraborty, H. S. & Gaarde, M. B. From single-cycle self-compressed filaments to isolated attosecond pulses in noble gases. Phys. Rev. A 77, 053814-1-10 (2008).
Gravel, J. F., Luo, Q., Boudreau, D., Tang, X. P. & Chin, S. L. Sensing of halocarbons using femtosecond laser-induced fluorescence. J. Anal. Chem. 76, 4799–4805 (2004).
Yao, J. P. et al. High-brightness switchable multiwavelength remote laser in air. Phys. Rev. A 84, 051802-1-5 (2011).
Rodriguez, M. et al. Triggering and guiding megavolt discharges by use of laser-induced ionized filaments. Opt. Lett. 27, 772–774 (2002).
Kovalenko, S. A., Dobryakov, A. L., Ruthmann, J. & Ernsting, N. P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 59, 2369–2384 (1999).
Kasparin, J. et al. White-light filaments for atmospheric analysis. Science 301, 61–64 (2003).
Courrol, L. C. et al. Color center production by femtosecond pulse laser irradiation in LiF crystals. Opt. Express 12, 288–293 (2004).
Dharmadhikari, J. A. et al. Writing low-loss waveguides in borosilicate (BK7) glass with a low-repetition-rate femtosecond laser. Opt. Commun. 284, 630–634 (2011).
Dharmadhikari, J. A. et al. Effect of chirp on the index contrast of waveguides written in BK7 glass with ultrashort laser pulses. Opt. Commun. 287, 122–127 (2013).
Dharmadhikari, J. A. et al. Axicon-based writing of waveguides in BK7 glass. Opt. Lett. 38, 172–174 (2013).
Mathur et al. Selective breaking of bonds in water with intense, 2-cycle, infrared laser pulses. J. Chem. Phys. 143, 244310–16 (2015).
Santhosh, C., Dharmadhikari, A. K., Alti, K., Dharmadhikari, J. A. & Mathur, D. Suppression of ultrafast supercontinuum generation in a salivary protein. J. Biomed. Opt. 12, 020510-1-3 (2007).
Chung, S. H. & Mazur, E. Surgical applications of femtosecond lasers. J. Biophoton. 2, 557 (2009).
D’Souza, J. S., Dharmadhikari, J. A., Dharmadhikari, A. K., Rao, B. J. & Mathur, D. Effect of intense, ultrashort laser pulses on DNA plasmids in their native state: strand breakages induced by in situ electrons and radicals. Phys. Rev. Lett. 106, 118101-1-4 (2011).
Dharmadhikari, A. K., Bharambe, H., Dharmadhikari, J. A., D’Souza, J. S. & Mathur, D. DNA damage by OH radicals produced using intense, ultrashort, long wavelength laser pulses. Phys. Rev. Lett. 112, 138105-1-5 (2014).
Meesat, R. et al. Cancer radiotherapy based on femtosecond IR laser-beam filamentation yielding ultra-high dose rates and zero entrance dose. PNAS 109, E2508–E2513 (2012).
Meesat, R. et al. Femtosecond laser pulse filamentation characterized by polymer gel dosimetry and Fricke dosimetry. J. Phys: Conf. Series 250, 012077-1-5 (2010).
Corkum, P. B., Rolle, C. & Srinivasan-Rao, T. Supercontinuum generation in gases. Phys. Rev. Lett. 57, 2268–2271 (1986).
Brodeur, A. & Chin, S. L. Ultrafast white-light continuum generation and self-focusing in transparent condensed media. J. Opt. Soc. Am. B 16, 637–650 (1999).
Liu, W. et al. Intensity clamping of a femtosecond laser pulse in condensed matter Opt. Commun. 202, 189–197 (2002).
Alfano, R. R. The Supercontinuum Laser Source (Springer-Verlag, Berlin, 2006).
Penzkofer, A., Seilmeier, A. & Kaiser, W. Parametric four-photon generation of picosecond light at high conversion efficiency. Opt. Commun. 14, 363–367 (1975).
Manassah, J. T., Baldeck, P. L. & Alfano, R. R. Self-focusing, self-phase modulation and diffraction in bulk homogeneous material. Opt. Lett. 13, 1090–1092 (1988).
Gaeta, A. L. Catastrophic collapse of ultrashort pulses. Phys. Rev. Lett. 84, 3582–3585 (2000).
Aközbek, N., Scalora, M., Bowden, C. M. & Chin, S. L. White-light continuum generation and filamentation during the propagation of ultra-short laser pulses in air. Opt. Commun. 191, 353–362 (2001).
Fang, X. J. & Kobayashi, T. Evolution of a super-broadened spectrum in a filament generated by an ultrashort intense laser pulse in fused silica. Appl. Phys. B 77, 167–170 (2003),
Kolesik, M., Katona, G., Moloney, J. V. & Wright, E. M. Physical factors limiting the spectral extent and band gap dependence of supercontinuum generation. Phys Rev Lett. 91, 043905-1-4 (2003).
Liu, W. et al. Femtosecond laser pulse filamentation versus optical breakdown in H2O. Appl. Phys. B 76, 215–229 (2003).
Geints, Yu. E. & Zemlyanov, A. A. Filamentation of high-power laser radiation in air and water: comparative analysis. Quant. Electronics 40, 121–126 (2010).
Dharmadhikari, A. K., Rajgara, F. A. & Mathur, D. Plasma effects and the modulation of white light spectra in the propagation of ultrashort, high-power laser pulses in barium fluoride. Appl. Phys. B 82, 575–583 (2006).
Thebergé, F., Liu, W., Simard, P. Tr., Becker, A. & Chin, S. L. Plasma density inside a femtosecond laser filament in air: Strong dependence on external focusing. Phys. Rev. E 74, 036406-1-7 (2006).
Lim, K., Durand, M., Baudelet, M. & Richardson, M. Transition from linear- to nonlinear-focusing regime in filamentation. Sci. Reports 4, 7217-1-8 (2014).
Dharmadhikari, A. K. et al. Dynamics of photothermally created microbubbles: Catastrophic cavitational collapse or slow dissolution? J. Phys. Chem. C 115, 6611–6623 (2011).
Sedelnikova, O. A., Pilch, D. R., Redon, C. & Bonner, W. M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2, 233–5 (2003).
Hoeijmakers, J. H. J. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
Cadet, J., Sage, E. & Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 571, 3–17 (2005).
Bristow, R. & Hill, R. P. Molecular and Cellular Radio Biology. The Basic Science of Oncology 4th Ed., eds. Tannock, I. F., Hill, R. P., Bristow, R. & Harrington, L. (McGraw-Hill, New York, 2005), Ch. 14.
Vogel, A. et al. Energy balance of optical breakdown in water at nanosecond to femtosecond time scales. App. Phy. B 68, 271–280 (1999).
Sacchi, C. A. Laser-inducd electric breakdown in water. J. Opt. Soc. Am. B 8, 337–341 (1991).
Minardi, S. et al. Energy deposition dynamics of femtosecond pulses in water. Appl. Phys. Lett. 105, 224104-1-4 (2014).
Sanche, L. Low energy electron-driven damage in biomolecules. Eur. Phys. J. D 35, 367–390 (2005).
Boudaiffa, B., Cloutier, P., Hunting, D., Huels, M. A. & Sanche, L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Ŝcience 287, 1658–1660 (2000).
Pan, X., Cloutier, P., Hunting, D. & Sanche, L. Dissociative electron attachment to DNA. Phys. Rev. Lett. 90, 208102-1-4 (2003).
Mathur, D. Biology-inspired AMO physics. J. Phys. B 48, 022001-1-22 (2015).
Nikogasyan, D. N., Oraevsky, A. A. & Rupasov, V. I. Two-photon ionizatino and dissociation of liquid water by powerful laser UV radiation. Chem. Phys. 77, 131–140 (1983).
Lian, R., Crowell, R. A. & Shkrob, I. A. Solvation and thermalization of electrons generated by above-the-gap (12.4 eV) two-photon ionization of liquid H2O and D2O. J. Phys. Chem. A 109, 1510–1520 (2005).
Mathur, D. & Hasted, J. B. Electron scattering by water and alcohol molecules. Chem. Phys. Lett. 34, 90–91 (1975).
Träutlein, D., Deibler, M., Leitenstorfer, A. & Ferrando-May, E. Specific local induction of DNA strand breaks by infrared multi-photon absorption. Nucleic Acid Res. 38, e14-1-7 (2010).
Kong, X. et al. Comparative analysis of different laser systems to study cellular responses to DNA damage in mammalian cells. Nucleic Acid Res. 37, e68-1-14 (2009).
von Sonntag, C. Free-radical Induced DNA Damage and its Repair. (Springer-Verlag, Berlin, 2006) p. 335.
Kumar, A., Pottiboyina, V. & Sevilla, M. D. Hydroxyl radical (OH*) reaction with guanine in an aqueous environment: a DFT study. J. Phys. Chem. B 115, 15129–15137 (2011).
Agnihotri, N. & Mishra, P. C. Reactivities of radicals of adenine and guanine towards reactive oxygen species and reactive nitrogen oxide species: OH* and . Chem. Phys. Lett. 503, 305–309 (2011).
Mathur, D., Dharmadhikari, A. K., Rajgara, F. A. & Dharmadhikari, J. A. Strong-field ionization of water by intense few-cycle laser pulses. Phys. Rev. A 78, 023414-1-6 (2008).
Acknowledgements
Financial support from the Department of Science and Technology is acknowledged by J.A.D. (Women Scientists Scheme) and D.M. (J. C. Bose National Fellowship). We also acknowledge the assistance rendered by Marilyn Sequeira in carrying out some of the sample preparation and gel measurements.
Author information
Authors and Affiliations
Contributions
D.M. and J.S.D′S. initiated and guided, respectively, the physics and biology aspects of the project. A.K.D. and J.A.D. developed the instrumentation and conducted the experiments along with H.B. and K.C.K. A.K.D. and J.A.D. developed the ultrashort laser experiments. K.C.K., H.B. and J.S.D′S. developed the DNA preparation and characterized the damage. K.D.R. carried out theoretical simulations. J.A.D., A.K.D. and D.M. interpreted the overall results and prepared the manuscript. All authors contributed to the discussion of the results and the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dharmadhikari, J., Dharmadhikari, A., Kasuba, K. et al. Optical control of filamentation-induced damage to DNA by intense, ultrashort, near-infrared laser pulses. Sci Rep 6, 27515 (2016). https://doi.org/10.1038/srep27515
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep27515
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.