Abstract
Acori Graminei Rhizoma is well known for the beneficial effects on CNS disorders in traditional medicine. Though it is frequently prescribed in formulations for brain tumors, the anti-glioma effect has not been examined. We used volatile oil of Acori Graminei Rhizoma (VOA) and human glioblastoma multiforme (GBM) cells in this study. We found that VOA exhibited greater growth suppression in p53 wild-type cells than p53 mutant cells and very low effect on fibroblasts and human glial HEB cells. Apoptosis was triggered by VOA with a caspase-dependent way in p53 wild-type A172 cells, while a caspase-independent way in p53 mutant U251 cells. Meanwhile, both A172 and U251 cells treated by VOA displayed autophagic features. Furthermore, p53 decrease was observed along with VOA-induced apoptosis and autophagy in A172 cells. VOA-induced autophagy was mediated through a p53/AMPK/mTOR signaling pathway in A172 cells, while an mTOR-independent signaling pathway in U251 cells. Finally, blockage of autophagy potentiated the proapoptotic effect in both A172 and U251 cells, indicating a protective role of autophagy in VOA-induced cell death. Together, VOA exhibited anti-tumor activity in human GBM cells and induced apoptotic cell death and protective autophagy, which is cell type specific and dependent on p53 status.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive malignant brain tumor. Patients suffering from GBM usually have extremely poor prognosis and the median survival is only 14 months1,2,3,4. Chemotherapy is preferably for brain cancer as malignant brain tumor cells may spread and grow within normal tissues, which limits the application of surgery5. However, the clinical effectiveness of the current chemotherapeutic agents is often restricted due to the blood-brain barrier (BBB), drug resistance and toxicity6,7,8. Therefore, the continued intensive investigation of new and innovative treatment strategies is urgently needed.
Substantial evidence has demonstrated that natural products-derived extracts and compounds can suppress tumor development and decrease the incidence and severity of cancer in human9. Induction of apoptosis is a classical way for natural products to exert their anti-tumor effect. Apoptosis is known as type I programmed cell death and generally characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms. It is well known that the caspase family in many cases involved in the apoptotic cell death, including mitochondria (caspase 9-caspase 3) way and death receptor (caspase 8-caspase 3) way. Furthermore, it has also been recently reported that many natural products participate in killing cancer cells by triggering autophagy, also called type II programmed cell death10. Autophagy is an evolutionarily cellular degradation process that involving in the delivery of cytoplasmic materials, such as aged proteins, mis-folded proteins or damaged organelles, for lysosome-dependent degradation following sequestration in double-membrane vesicle (autophagosomes)11,12. In addition, natural products may also result in a protective autophagy during induction of cancer cell death, which is a process naturally regarded as cellular stress clearance10. Apoptosis and autophagy may be interacted with each other and regulated by some common proteins, such as atg5, Bcl-2 and p53. For instance, p53, a tumor suppressor, is well known for coordinating apoptosis to preserve genomic stability and prevent tumor formation. Recently reports have also suggested the involvement of p53 in the autophagic pathway.
Acori Graminei Rhizoma, the dry rhizome of acorusgramineus solander (Araceae), has been used in Chinese medicine for more than hundreds of years. Traditionally, Acori Graminei Rhizoma is mainly used for central nervous system (CNS) disorders. Investigations in the past decade mainly focused on its action in ameliorating learning and memory deficits and improving the cognitive function13,14. Meanwhile, Acori Graminei Rhizoma is also frequently prescribed for the treatment of brain tumor in some Chinese medicine formulations. However, the anti-glioma effect of Acori Graminei Rhizoma has not been examined.
Volatile oil of Acori Graminei Rhizoma (VOA) has been reported to be the main bioactive components15. In the current study, we first determined the anti-glioma activity of VOA. Then, the role of apoptosis and autophagy played in the VOA’s anti-tumor effect was further investigated. And finally, the influence of tumor suppressor p53 on both apoptosis and autophagy triggered by VOA was specially examined.
Results
VOA induced differential growth inhibitory effect on p53 wild-type and p53 mutant human GBM cells
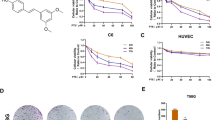
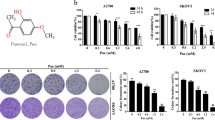
The cytotoxic effect of VOA with different concentrations on GBM cells including A172, U87, U251 and U118 cells were determined at 48 h and 72 h by Sulforhodamine B (SRB) assay. In addition, NIH/3T3 embryonic fibroblast cells and human glia HEB cells were also used to determine the cytotoxic effect of VOA in normal cells. As shown in Fig. 1A, it’s interesting to find that A172 and U87 cells, which are p53 wild-type cell lines, were more sensitive to VOA’s cytotoxicity than U251 and U118 cells, which are p53 mutant cell lines. Additionally, VOA treatment induced relatively low cytotoxicity in NIH/3T3 cells and HEB cells compared to that in human GBM cells. Furthermore, the clonogenic assay performed with a sustained treatment of A172 and U251 cells for two weeks also showed that the inhibition concentrations of VOA in A172 cells were significantly lower than that in U251 cells (Fig. 1B).
VOA induced differential cell death in human glioma cells and normal cells.
(A) p53 wild-type cells (A172 and U87), p53 mutant cells (U251 and U118) and normal cells (HEB and NIH/3T3) were treated with 0, 25, 50, 75, 100, 150, 175, 200 and 250 μg/ml VOA for 48 or 72 h and cell viability were determined by SRB assay. (B) Colonies in A172 and U251 cells were treated with indicated concentrations of VOA and allowed to grow for 2 weeks before stained with 0.005% crystal violet. Adjacent picture depicts the crystal violet-stained colonies and bar graph indicated the cloning efficiency compared with untreated control. Values represent mean ± SD. *p < 0.05 compared with control.
VOA induced apoptosis in both A172 cells and U251 cells
To identify whether VOA-induced cytotoxicity was due to apoptosis, we evaluated the inhibitory effect of VOA with Annexin V/PI staining analysis by flow cytometry. The result in Fig. 2A showed that a significant increase of apoptotic cells was induced by VOA treatment after 48 h in both A172 and U251 cells. In particular, the percentage of apoptotic cells increased from 8.4% to 21.7% in 100 μM of VOA treated-A172 cells, while from 11.4% to 28.0% in 200 μM of VOA treated-U251 cells. Furthermore, we used Hoechst 33342 staining to detect the morphologic change of apoptotic cells. As shown in Fig. 2B, both VOA treated-A172 and U251 cells exhibited much more cells with condensed and fragmented nuclei than those treated with vehicle alone.
Apoptosis occurred in both A172 and U251 cells after VOA treatment.
A172 and U251 cells were treated with indicated concentrations of VOA for 48 h. (A) Apoptotic cells were quantified by flow cytometry after stained with FITC-conjugated Annexin V and PI. (B) Morphologic change of apoptotic cells was evaluated by Hoechst 33342 staining. Etoposide (25 μM) was used as positive control. The scale bar is 50 μm. Values represent mean ± SD. *p < 0.05.
VOA triggered caspase-dependent apoptosis in A172 cells but caspase-independent apoptosis in U251 cells
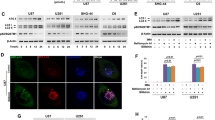
To further determine the potential signaling pathway involved in the VOA-induced apoptosis of A172 and U251 cells, cell viability was checked by SRB assay after pre-treatment with pan-caspase inhibitor Z-VAD-FMK16. As shown in Fig. 3A, exposure of A172 cells to VOA (100 μg/ml) with Z-VAD-FMK mostly prevented VOA-provoked cell death while this phenomenon did not happen in 200 μg/ml of VOA-treated U251 cells. These results suggest that VOA may trigger caspase-dependent apoptosis in A172 but caspase-independent apoptosis in U251 cells. To further confirm our deduction, caspase-related apoptotic proteins were determined. Western blotting analysis results in Fig. 3B revealed that VOA induced a concentration-dependent apoptosis way in A172 cells with the cleavage of caspase 3, caspase 8 and caspase 9. Meanwhile, the ratio of Bax/Bcl-2 also increased after VOA treatment for 48 h. However, neither cleavage of caspase-related proteins nor ratio of Bax/Bcl-2 significantly increased in U251 cells. These data indicate that caspase 3-mediated intrinsic and extrinsic apoptosis exists in VOA-treated A172 cells, while VOA-treated U251 cells may go to apoptosis in other ways which are still waiting to explore.
VOA induced caspase-dependent apoptosis in A172 cells and caspase-independent apoptosis in U251 cells.
(A) Cells were pretreated with pan-caspase inhibitor Z-VAD-FMK (25 μM) for 2 h and then treated with VOA (100 μg/ml for A172 cells and 200 μg/ml for U251 cells) for 48 h. The cell viability was determined by SRB assay. Values represent mean ± SD. *p < 0.05. (B) A172 and U251 cells were treated with indicated concentrations of VOA for 48 h. Then, expression of apoptosis-related proteins including Bax, Bcl-2, pro-caspase 3, cleaved caspase 3, 8 and 9 was determined by Western blotting. The blots were a representative of three independent experiments.
VOA activated autophagy process in both A172 and U251 cells
To determine whether autophagy is involved in VOA-induced cell death, we firstly analyzed the effect of VOA on LC3-II/I protein, which is considered as a determinate maker of autophagy activation. The results (Fig. 4A) showed that VOA increased the ratio of LC3-II/I in both A172 and U251 cells. To further ascertain the autophagosome formation in VOA-treated cells, the changes of the following major autophagy related makers, p62, beclin-1 and atg5, were analyzed by Western blotting after 48 h treatment. As a result, p62 was significantly decreased in a concentration-dependent way, while atg5 and beclin1 were remarkably increased. Then, we further transfected GFP-LC3 into both A172 and U251 cells. As shown in Fig. 4B, a diffuse localization of LC3 fluorescence was observed in both untreated A172 and U251 cells, whereas a punctate pattern of LC3 fluorescence was detected in both VOA-treated cells. Further acridine orange staining (Fig. 4C) showed that there was obvious formation of acidic vesicular organelles (AVOs) in VOA-treated A172 (100 μM) and U251 (200 μM) cells. Together, these data strongly indicate that VOA induced autophagy in both A172 and U251 cells.
Autophagy was triggered in both A172 and U251 cells by VOA.
(A) A172 and U251 cells were treated with indicated concentrations of VOA for 48 h. Then, expression of autophagy related proteins including LC3II/I, p62, atg 5 and beclin 1 was detected by Western blotting. The blots were a representative of three independent experiments. (B) A172 and U251 cells were transfected with GFP-LC3 for 6 h and then treated with VOA (100 μg/ml for A172 cells and 200 μg/ml for U251 cells) for 48 h. A punctate distribution of LC3 in both cells was observed by fluorescence microscope (400×). The scale of bar is 100 μm. (C) A172 and U251 cells were treated with indicated concentrations of VOA for 48 h, then stained by acridine orange and observed under fluorescence microscope (200×). The scale of bar is 100 μm.
VOA-mediated AMPK/mTOR signaling pathway in A172 cells and AMPK/mTOR-independent pathway in U251 cells
To elucidate the potential molecular mechanisms leading to the VOA-induced autophagy, we evaluated whether AMPK and mTOR were involved in this autophagy flux in both A172 and U251 cells. Our results (Fig. 5A) showed that VOA enhanced AMPK phosphorylation in A172 cells. Moreover, it inhibited the activation of the AMPK downstream targets mTOR and p70 S6K by decreasing their phosphorylation, suggesting the AMPK/mTOR signaling pathway may contribute to the activation of autophagy by VOA in A172 cells. However, we found that VOA could induce autophagy via the activation of AMPK pathway without inhibiting either phosphorylation of mTOR or its substrates p70 S6K in U251 cells (Fig. 5B). This indicated that a bypassing mechanism of AMPK signaling pathway independent of mTOR suppression may be involved in the VOA’s activation of autophagy in U251 cells. ULK1 protein kinase is a key initiator of the autophagic process and plays a critical role in the AMPK and mTORC1 mediated autophagy regulation17,18. Thus, we determined the effect of VOA on ULK1-Ser555 in A172 and U251 cells. As a result, ULK1-Ser555 was activated in U251 cells but not in A172 cells (Fig. 5C).
VOA induced AMPK/mTOR-dependent autophagy in A172 cells and AMPK/mTOR-independent autophagy in U251 cells.
A172 (A,C) and U251 (B,D) cells were treated with indicated concentrations of VOA for 48 h. Then, expression of p-AMPK, AMPK, p-mTOR, mTOR, p-p70S6K, p70S6K, p-ULK1-Ser555 and ULK1 was detected by Western blotting. The blots were a representative of three independent experiments.
Recent reports also showed autophagy depends on the activation of MAP kinases signaling pathway19. To investigate whether the activation of MAP kinases signaling pathway contributes to the induction of autophagy in U251 cells, the expression of the phosphorylated forms of ERK1/2, P38 MAPK and JNK were examined by immunoblotting. As shown in Figure S3, VOA induced a significant activation of ERK1/2, P38 MAPK and JNK in a concentration-dependent way in U251 cells. Thus, it is possible that VOA-induced autophagy in U251 cells may also be regulated by MAP kinase signaling pathway.
VOA-induced apoptosis and autophagy related to the decrease of p53 level in A172 cells
The human tumor suppressor protein p53, which transactivates many of pro-apoptotic and cell cycle arresting-related genes, is well recognized for its ability to induce apoptosis20. Recent reports have also suggested a novel function of p53 in regulating autophagy21,22,23. Thus, to investigate the role of p53 in VOA-triggered apoptosis and autophagy, we first determined p53 expression level after VOA treatment in both A172 and U251 cells. As a result, p53 level was negatively correlated with the concentration of VOA over the range of 0–100 μg/ml after 48 h treatment in A172 cells. Moreover, VOA decreased p53 expression level starting from 6 h treatment in A172 cells (Fig. 6A). This result was also confirmed by the observation that a concentration- and time-dependent p53 decrease occurred in U87 cells after VOA treatment (Figure S4A). In contrast, no p53 change was observed after VOA treatment with different concentrations and time points in U251 cells (Fig. 6B). The results implicated that VOA may reduce p53 level in p53 wild-type GBM cells compared with no total p53 alteration in p53 mutant GBM cells. Previous reports showed that β-asarone and β-elemene, two main components of VOA, exhibited p53 inducing effect24,25. Then, we determined the effect of VOA and these main components on p53 expression side by side in A172 cells. As shown in Figure S5, VOA decreased p53 expression as determined previously. Beta-asarone (25 and 50 μM) and β-elemene (25 μM) alone significantly increased p53 expression and β-elemene (50 μM) alone exhibited no obvious effect on p53 expression. However, the combination of β-asarone (25 μM) and β-elemene (25 μM) remarkably reversed the up-regulation of p53 expression by these two compounds alone. Moreover, the combination of β-asarone (50 μM) and β-elemene (50 μM) even slightly down-regulated p53 expression compared to control. These results indicated that the effect of main components in VOA on p53 expression is concentration-dependent. When these components used as a high concentration alone or as combination, p53 expression may be reversed or even down-regulated just as the effect exhibited by VOA.
VOA-induced apoptosis and autophagy in A172 cells were associated with the decrease in p53 level.
A172 (A) and U251 (B) cells were treated with indicated concentrations of VOA for 48 h or with VOA (100 μg/ml for A172 cells and 200 μg/ml for U251 cells) for indicated time points. Then, expression of p53 was detected by Western blotting. The blots were a representative of three independent experiments. (C) A172 cells were pre-treated with or without PFT-α (10 μM) for 2 h or transfected with si-p53 or si-CTRL for 6 h and then treated with VOA (100 μg/ml) for 48 h. Expression of LC3II/I, p62, cleaved caspase 3, p-mTOR, mTOR, p-AMPK, AMPK. The blots were a representative of three independent experiments.
To further determine the role of p53 down-regulation after VOA treatment in A172 cells, we inhibited p53 level with pifithrin-α (PFT-α), which was validated by the inhibition of p53 responsive gene p21 expression (Figure S6) or knocked it down with a specific p53 small interfering RNA (si-p53). Figure 6C showed that VOA-induced cleaved caspase 3 and LC3II/I formation further increased, while VOA-down-regulated p62 expression further reduced after PFT-α pre-treatment or si-p53 transfection. These results indicated that there is a direct impact of p53 decrease on VOA-induced apoptotic and autophagic processes. In addition, it is reported that p53 mediates autophagy through an AMPK/mTOR-dependent pathway26, leading us to hypothesize that AMPK/mTOR may be involved in p53 loss-activated autophagy in VOA treated-A172 cells. As showed in Fig. 6B, VOA induced-decrease of p-mTOR and increase of p-AMPK were remarkably augmented after PFT-α pretreatment or si-p53 transfection in A172 cells. Furthermore, we confirmed the above observation in U87 cells. The result showed that VOA induced-increase of LC3II/I, cleaved caspase 3 and p-AMPK and decrease of p-mTOR were also augmented in U87 cells after PFT-α pretreatment (Figure S4B). Taken together, these results revealed that p53 inhibition or knockdown could enhance the induction of apoptosis and autophagy by VOA in human glioma cells harboring wild-type p53. Moreover, p53-mediated AMPK/mTOR pathway may play a critical role in VOA-induced autophagy in A172 cells.
Inhibition of autophagy switches it to apoptosis in both A172 and U251 cells with VOA intervention
There is a complex relationship between autophagy and apoptosis in the modulation of cellular survival responses22,23,27. To investigate the role of autophagy in VOA-induced cell death, we used 3-methyladenine (3-MA) or chloroquine (CQ) to inhibit the autophagy caused by VOA. The results of SRB assay showed in Fig. 7A demonstrated that inhibition of autophagy by both 3-MA and CQ is capable of promoting the VOA-induced cell death in both A172 and U251 cells. Next, Western blotting analysis showed that co-treatment VOA with 3-MA or CQ caused an increase of caspase 3 cleavage in A172 cells (Fig. 7B), suggesting that inhibition of autophagy promotes the caspase-dependent apoptosis in p53 wild-type cells. However, there is no change of cleaved caspase 3 in U251 cells, further suggesting that a caspase-independent apoptosis is triggered by VOA in U251 cells. These results were also confirmed by immunocytofluorescent staining. Figure 7C showed that co-treatment of VOA with 3-MA caused higher fluorescent level of activated caspase 3 in A172, while no remarkably change was observed in U251 cells. These results indicate that VOA-induced autophagy is a protective mechanism and blockage of autophagy aggravates the VOA-induced apoptosis. In Figure S7, flow cytometry analysis showed that apoptotic cell death increased from 20.1% to 30.4% in 100 μg/ml of VOA-treated A172 cells and from 19.8% to 31.0% in 200 μg/ml of VOA-treated U251 cells after the incorporation of 3-MA. However, 3-MA alone in both A172 and U251 also led to increased apoptotic cells compare to control. Therefore, based on this result, the potential role of 3-MA-induced apoptosis couldn’t be excluded from the apoptosis inducing effect of VOA and 3-MA co-treatment.
Inhibition of autophagy enhanced VOA-induced apoptosis.
A172 and U251 cells were pretreated with or without 3-MA (2.5 mM) or CQ (10 μM) for 2 h and then treated with VOA (100 μg/ml) for 48 h. (A) Cell viability of A172 and U251 cells were measured by SRB assay. Values represent mean ± SD. *p < 0.05. (B) Expression of LC3II/I and cleaved caspase 3 was detected by Western blotting. The blots were a representative of three independent experiments. (C) A172 and U251 cells were pretreated with or without 3-MA (2.5 mM) for 2 h and then treated with VOA (100 μg/ml) for 48 h. Immunofluorescence staining of cleaved caspase 3 was observed by fluorescence microscope (400×). The scale of bar is 100 μm.
Discussion
Despite considerable advances in brain cancer therapy in the past decade, the prognosis for patients having glioblastoma remains extremely poor28,29. The combined treatment based on radiotherapy and temozolomide is considered to be the optimal treatment for patients with glioblastoma, which doubles the 2-year survival rate to 27%. However, the efficacy of temozolomide is limited due to its drug resistance and toxic side effects30,31. Thus, it is crucial to develop new chemotherapeutic agents for treating patients with glioblastoma.
In recent years, Chinese herbal medicine has received extensive attention as the source of new drugs for anti-cancer therapy32. Acori graminei Rhizoma is traditionally used for CNS disorders in China for more than hundreds of years. Though it is frequently prescribed in the formulations for the treatment of brain tumor, the effect is still not directly validated and the potential mechanisms are also unclear. The volatile oil of Acori graminei Rhizoma (VOA) is believed to be the main active components. The major compounds existing in the VOA are detectable in the rat brain tissue after oral administration33. And importantly, they are reported to inhibit the function and expression of P-glycoprotein and improve the permeability of BBB34,35.
In the present study, we first investigated the growth inhibitory effect of VOA on different human GBM cell lines and normal cell lines. VOA exhibited low cytotoxicity to normal cell lines, while it induced significant cytotoxicity to human GBM cell lines. Interestingly, the cytotoxicity of VOA seems to be related to p53 status as p53 wild-type GBM cells (A172 and U87) are more sensitive to p53 mutant GBM cells (U251 and U118). Furthermore, the result of clonogenic assay also supported our deduction.
Natural products usually exert their anticancer effects by triggering apoptosis and autophagy36,37. Thus, we first determined the role of apoptosis in VOA induced cell death. The results of Annexin V/PI staining and Hoechst 33342 staining demonstrated that apoptosis was induced by VOA in both A172 cells and U251 cells. However, it’s interesting that pre-treatment with pan-caspase inhibitor Z-VAD-FMK mostly prevented VOA-provoked cell death in A172 cells, whereas this phenomenon did not happen in U251 cells, suggesting that caspase-dependent and independent apoptotic pathways were triggered in A172 and U251 cells, respectively. Apoptosis related proteins caspase 3, caspase 8 and caspase 9 play extremely important roles in the apoptotic signaling ways. Caspase 3, a key executioner in the apoptotic machinery, cleaves many proteins indispensable for cell survival and is activated through cleavage into two fragments (17 kDa and 19 kDa) by caspase 8 and/or caspase 9 during apoptosis38. Caspase 8 and caspase 9 play crucial roles in the induction of apoptosis through death receptor-mediated (extrinsic) and the mitochondrial (intrinsic) pathways, respectively39. Moreover, the balance between expression levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax is also critical for cell survival and death40, where increase of the Bax/Bcl-2 ratio activates the release of cytochrome C from mitochondria and subsequently activates the intrinsic apoptotic pathway22. Therefore, the effect of VOA on these apoptosis related proteins was further determined in this study. Our results demonstrated that VOA obviously increased the cleavage of apoptosis-executive caspase 3, caspase 8 and caspase 9 and also contributed to a significantly increased Bax/Bcl-2 ratio, suggesting that the mitochondrion-dependent intrinsic apoptosis pathway and receptor-mediated extrinsic pathway participated in VOA associated cytotoxicity in A172 cells. However, the results obtained from U251 cells demonstrated that VOA neither promoted the cleavage of caspase family proteins, nor changed the Bax/Bcl-2 ratio. A possible explanation for this is the different genetic background of these two cell models. It is reasonable to assume that VOA-induced apoptotic signaling pathway may be dependent on p53 deficient/mutant status.
As a complementary mechanism of cell death, autophagy is also involved in the anti-cancer activity of a wide variety of natural products41. We then determined whether autophagy was triggered in VOA-induced cell death. Our study revealed that VOA remarkably increased the ratio of LC3-II/I, atg5 and beclin1 and decreased p62 levels in both A172 and U251 cells, indicating that autophagy was induced by VOA in these two different cell lines. This effect was further validated by observation with fluorescence microscope after GFP-LC3 transfection and AO staining, where VOA significantly increased the punctate pattern of LC3 fluorescence and the formation of AVOs. It’s well known that multiple upstream signaling pathways are involved in the induction of autophagy, including beclin-1 (atg6), AMPK/mTOR, calcium signaling, endoplasmic reticulum-stress and Ras/Raf/MAPK pathway42. Among them, AMPK/mTOR plays a central role in the regulation of autophagy by integrating and coordinating different sensory inputs from upstream factors and then phosphorylating Atg proteins31,43. In our study, it’s noticeable that VOA induced autophagy via AMPK/mTOR signaling pathway in A172 cells but independently of mTOR inhibition in U251 cells. Similarly, previous investigation also reported that fangchinoline induced autophagy via the activation of the AMPK pathway without inhibiting either phosphorylation of mTOR26. ULK1-Ser555 has been reported to be phosphorylated by AMPK directly44. Our further result demonstrated that VOA-induced autophagy in U251 cells may be mediated by AMPK via directly phosphorylating ULK1-Ser555, which is consistent with the result that VOA-induced autophagy is independent of mTOR in U251. Meanwhile, our result also indicated that ULK1-Ser555 was not involved in VOA-induced autophagy in A172 cells, in which the AMPK/mTOR signaling pathway played a critical role as reflected in Fig. 5A.
It has been reported that p53 was found to play critical roles in the regulation of not only apoptosis, but also autophagy21,43,45. In our study, VOA remarkably decreased p53 level in a concentration- and time-dependent manner in A172 and U87 cells, while no significant change was observed in U251 cells. Recent investigations revealed that p53 decrease is closely related to apoptosis and autophagy induced by different stimuli46. Thus, we further evaluated the role of p53 decrease in VOA-induced apoptosis and autophagy. Similarly, our results showed that augment of both apoptosis and autophagy induced by VOA could be achieved by co-treatment of p53 inhibitor PFT-α in both A172 and U87 cells or transfection of p53 siRNA in A172 cells. Meanwhile, p53 decrease further enhanced the VOA-activated AMPK/mTOR in both A172 and U87 cells, strongly suggesting that VOA induced autophagy in wild-type GBM cells by p53-mediated AMPK/mTOR pathway. These results are in accordance with previous reports that docosahexaenoic acid (DHA)47 and mimulone38 induced autophagy via p53/AMPK/mTOR signaling in human cancer cells with wild-type p53.
In many cases, apoptosis and autophagy were simultaneously induced by natural products46,47. The interconnection between them is complex and varies with the nature of stimulus and cell type. As autophagy is a process that consumes cellular components and generates energy, it may sensitize cells to apoptosis by acting as an energy source or suppress apoptosis as protective autophagy by clearing damaged organelles under stress condition48. In the present study, blockage of autophagy by 3-MA or CQ augmented apoptosis induced by VOA in both A172 and U251 cells, indicating that VOA-induced autophagy might play a protective role.
Conclusion
In this study, VOA exhibited obvious growth inhibitory effect on human malignant glioma cells, which seems to be closely related to p53 status. Apoptotic cell death and protective autophagy were induced by VOA in both A172 and U251 cells. Moreover, the underlying mechanisms are cell type specific and dependent on p53 status. Further study could be ongoing to explore whether there are some ingredients in the Acori Graminei Rhizoma-containing formulations that regulate autophagy to sensitize tumor cells to anticancer therapy.
Materials and Methods
Materials
VOA was extracted from Acori Graminei Rhizoma by a well-established procedure in our lab (the details described in Supplementary Methods) and stored in −80 °C before use. Four major components (β-asarone, tatarine A, β-elemene and zierone) were identified by HPLC-MS in this extract (Figures S1 and S2). The antibodies against caspase 3, cleaved caspase 3, cleaved caspase 9, LC 3II/I, atg 5 and beclin-1 were obtained from Cell Signaling Technology (Boston, MA, USA). SQSTM1/p62 antibody was obtained from Santa Cruz Biotechnology (CA, USA). The antibodies against β-actin and rabbit IgG were obtained from Sigma-Aldrich (St. Louis, MO, USA). The antibodies against p53 and cleaved caspase 8 were obtained from Wanlei Biotechnology (Shenyang, China). Other chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) unless indicated otherwise.
Cell culture
Human glioblastoma multiforme (GBM) cells A172, U87 and U251 and mouse embryonic fibroblast NIH/3T3 cells were obtained from Kunming Institute of Zoology, CAS. Human GBM U118 and human glial cells HEB were obtained from Zhongshan University. All the cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, USA) and 1% penicillin/streptomycin (Invitrogen, USA) at 37 °C in a humidified 5% CO2 atmosphere.
Measurement of cell viability
Cell viability was evaluated by sulforhodamine B (SRB) assay (Sigma-Aldrich, St. Louis, MO, USA), which was based on the measurement of cellular protein content49,50 and stained with 0.4% SRB for 30 min. The protein-bound dye is dissolved in 10 mM Tris base solution for OD determination at a wavelength of 490 nm using a multi-well spectrophotometer microplate reader (Biotek, Winooski, VT, USA). Cell viability was expressed as a percentage of that of the control (untreated) cells.
Clonogenic assay
Cells were seeded in a 6-well plate and treated with different concentrations of VOA. Colonies were allowed to form for two weeks and medium with VOA or vehicle was replaced per 3 days. At the end of treatment, cells were fixed in 100% methanol and stained with 0.005% crystal violet. Finally, images were captured by a CCD camera and the colonies were counted.
Flow cytometry
Cells were seeded into 6-well plates at a density of 1.6 × 105 cells/well. After drug treatment, cells were harvested, washed and resuspended in the binding buffer containing annexin V and propidium iodide (PI). After incubation at room temperature in the dark for 15 min, the stained cells were subjected to a BD LSRFortessa Cell Analyzer (BD Biosciences, San Jose, CA, USA) with fluorescence emission at 530 nm and 575 nm and excitation at 488 nm. Data were analyzed using Flow Jo 7.6.1 software (Tree Star, Inc., Ashland, OR, USA).
Hoechst 33342 staining
As previously described51,52, VOA-treated cells were stained with apoptosis-specific dye Hoechst 33342. After treatment at 6-well plates for 48 h, cells were washed with 1×PBS for 3 times. Then, Hoechst 33342 dissolving in 1×PBS was added into each well. The plates were kept at room temperature for 10 min and avoided from light. Finally, the plates were washed with 1×PBS again and images were captured on a Zeiss fluorescence microscope (Carl Zeiss, Germany).
GFP-LC3 transfection
As previously described53, cells were seeded into a 6-well plate at a density of 1.0 × 105 cells/well and allowed to reach approximately 40–50% confluence on the day of transfection. Cells were then transfected with 4 μg plasmid of green fluorescent protein-microtubule-associated protein 1 light-chain 3 (GFP-LC3) using transfection reagent Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. After a 6 hour medium incubation, the transfection medium was removed and the cells were incubated in fresh medium for 24 h, followed by the treatment with different concentrations of VOA. The accumulation of intense punctuate aggregations of GFP-LC3 was observed and recorded under a Zeiss fluorescence microscope (Carl Zeiss, Germany).
Acridine orange staining
As previously described54,55, cells were seeded into 6-well plate at a density of 1.6 × 105 cells/well. After treatment with VOA, cells were washed twice with 1×PBS, followed by incubation with 0.005 μg/ml acridine orange (Invitrogen, USA) at 37 °C for 15 min. The stained cells were washed with 1×PBS and the fluorescence signal was detected and recorded under a Zeiss fluorescence microscope (Carl Zeiss, Germany).
RNA interference
Small interfering RNAs targeting p53 (si-p53) and a control siRNA (si-CTRL) were obtained from sigma (St. Louis, MO, USA). Cells were seeded into a 6-well plate at a density of 1.0 × 105 cells/well and allowed to reach approximately 50% confluence on the day of transfection. Cells were then transfected with 50 nM siRNA using transfection reagent Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. After a 6 hour antibiotic-free medium incubation, the transfection medium was removed and the cells were incubated in fresh medium for 24 h, followed by further drug treatments.
Western blotting analysis
The cellular proteins were extracted from A172 and U251 cells in ice-cold RIPA buffer (Cell Signaling Technologies, USA) supplemented with 1% (v/v) protein inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride (PMSF). Thirty micrograms of the cellular proteins were resolved by electrophoresis in 12% SDS-polyacrylamide gel and subsequently transferred to polyvinylidene difluoride (PVDF) membrane. Following 1 h incubation in a fresh TBS buffer containing 0.1% Tween-20 and 5% BSA, the blots were probed with specific primary antibodies. After incubation with the relevant secondary antibodies, the reactive bands were identified using an enhanced chemiluminescence (ECL) detection reagent (GE Healthcare, Sweden). The concentration of the loaded cellular proteins was normalized against the internal control β-actin and then the value was expressed as each normalized data relative to control.
Statistical analysis
All data were presented as mean ± SD for three independent experiments. Statistical analysis was performed by two-tail Student’s t-test. A p-value of less than 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Chen, L. et al. Volatile Oil of Acori Graminei Rhizoma-Induced Apoptosis and Autophagy are dependent on p53 Status in Human Glioma Cells. Sci. Rep. 6, 21148; doi: 10.1038/srep21148 (2016).
References
Castellano, A. et al. Evaluation of low-grade glioma structural changes after chemotherapy using DTI-based histogram analysis and functional diffusion maps. Eur. Radiol., 10.1007/s00330-015-3934-6 (2015).
Chamdine, O., Broniscer, A., Wu, S., Gajjar, A. & Qaddoumi, I. Metastatic low-grade gliomas in children: 20 years’ experience at St. Jude Children’s Research Hospital. Pediatr. Blood Cancer, 10.1002/pbc.25731 (2015).
Chen, M. et al. Automatic estimation of midline shift in patients with cerebral glioma based on enhanced voigt model and local symmetry. Australas. Phys. Eng. Sci. Med., 10.1007/s13246-015-0372-3 (2015).
Finocchiaro, G. & Pellegatta, S. Novel mechanisms and approaches in immunotherapy for brain tumors. Discov. Med. 20, 7–15 (2015).
Giese, A., Bjerkvig, R., Berens, M. E. & Westphal, M. Cost of migration: invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 21, 1624–1636, 10.1200/JCO.2003.05.063 (2003).
Wang, X. et al. NF-kappaB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol. Lett. 9, 2586–2590, 10.3892/ol.2015.3130 (2015).
Blacklock, J. B. et al. Drug streaming during intra-arterial chemotherapy. J. Neurosurg. 64, 284–291, 10.3171/jns.1986.64.2.0284 (1986).
Elliott, P. J. et al. Unlocking the blood-brain barrier: a role for RMP-7 in brain tumor therapy. Exp. Neurol. 141, 214–224, 10.1006/exnr.1996.0156 (1996).
La Vecchia, C. & Tavani, A. Fruit and vegetables and human cancer. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation 7, 3–8 (1998).
Wang, N. & Feng, Y. Elaborating the role of natural products-induced autophagy in cancer treatment: achievements and artifacts in the state of the art. BioMed research international 2015, 934207, 10.1155/2015/934207 (2015).
Shi, Y. et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 11, 769–784, 10.1080/15548627.2015.1034411 (2015).
Lee, S. W. et al. The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett. 360, 195–204, 10.1016/j.canlet.2015.02.012 (2015).
Zhang, H. et al. Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. J. Pharm. Pharmacol. 59, 301–309, 10.1211/jpp.59.2.0016 (2007).
Kim, J. H., Hahm, D. H., Lee, H. J., Pyun, K. H. & Shim, I. Acori graminei rhizoma ameliorated ibotenic acid-induced amnesia in rats. Evid. Based Complement. Alternat. Med. 6, 457–464, 10.1093/ecam/nem158 (2009).
Cho, J. et al. NMDA recepter-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci. 68, 1567–1573 (2001).
Kim, A. D. et al. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 4, e750, 10.1038/cddis.2013.273 (2013).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461, 10.1126/science.1196371 (2011).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141, 10.1038/ncb2152 (2011).
Liu, J. et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem. Biophys. Res. Commun. 463, 262–267, 10.1016/j.bbrc.2015.05.042 (2015).
Zhang, J., Yang, Y., Lei, L. & Tian, M. Rhizoma Paridis Saponins Induces Cell Cycle Arrest and Apoptosis in Non-Small Cell Lung Carcinoma A549 Cells. Med. Sci. Monit. 21, 2535–2541, 10.12659/MSM.895084 (2015).
Tasdemir, E. et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687, 10.1038/ncb1730 (2008).
Shen, S., Zhang, Y., Wang, Z., Liu, R. & Gong, X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int. J. Biol. Sci. 10, 212–224, 10.7150/ijbs.8056 (2014).
Levine, B. & Abrams, J. p53: The Janus of autophagy? Nat. Cell Biol. 10, 637–639, 10.1038/ncb0608-637 (2008).
Li, J., JunYu, Liu, A. & Wang, Y. beta-Elemene against human lung cancer via up-regulation of P53 protein expression to promote the release of exosome. Lung Cancer 86, 144–150, 10.1016/j.lungcan.2014.08.015 (2014).
Liu, L. et al. beta-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine 20, 512–520, 10.1016/j.phymed.2012.12.008 (2013).
Wang, N. et al. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br. J. Pharmacol. 164, 731–742, 10.1111/j.1476-5381.2011.01349.x (2011).
Ning, Y., Li, Z. & Qiu, Z. FOXO1 silence aggravates oxidative stress-promoted apoptosis in cardiomyocytes by reducing autophagy. J. Toxicol. Sci. 40, 637–645, 10.2131/jts.40.637 (2015).
Weathers, S. P. & Gilbert, M. R. Advances in treating glioblastoma. F1000prime reports 6, 46, 10.12703/P6-46 (2014).
Lacroix, M. et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection and survival. J. Neurosurg. 95, 190–198, 10.3171/jns.2001.95.2.0190 (2001).
Messaoudi, K., Clavreul, A. & Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug discovery today 20, 899–905, 10.1016/j.drudis.2015.02.011 (2015).
Grieco, A. et al. Severe cholestatic hepatitis due to temozolomide: an adverse drug effect to keep in mind. Case report and review of literature. Medicine (Baltimore) 94, e476, 10.1097/MD.0000000000000476 (2015).
Ho, J. W., Leung, Y. K. & Chan, C. P. Herbal medicine in the treatment of cancer. Curr. Med. Chem. Anticancer Agents 2, 209–214 (2002).
Hongmei Tang, R. L. Determination of chemical components in Rhizoma Acori graminei after permeability of rat blood brain barrier. Traditional Chinese Medicine research 18, 40–41 (2002).
Meng, X. et al. Reversing P-glycoprotein-mediated multidrug resistance in vitro by alpha-asarone and beta-asarone, bioactive cis-trans isomers from Acorus tatarinowii. Biotechnol. Lett. 36, 685–691, 10.1007/s10529-013-1419-8 (2014).
Hu, Y., Yuan, M., Liu, P., Mu, L. & Wang, H. [Effect of Acorus tatarinowii schott on ultrastructure and permeability of blood-brain barrier]. Zhongguo Zhong Yao Za Zhi 34, 349–351 (2009).
Tan, W. et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 6, 27, 10.1186/1749-8546-6-27 (2011).
Cragg, G. M. & Newman, D. J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 100, 72–79, 10.1016/j.jep.2005.05.011 (2005).
An, H. K. et al. Mimulone-induced autophagy through p53-mediated AMPK/mTOR pathway increases caspase-mediated apoptotic cell death in A549 human lung cancer cells. PLoS One 9, e114607, 10.1371/journal.pone.0114607 (2014).
Zaman, S., Wang, R. & Gandhi, V. Targeting the apoptosis pathway in hematologic malignancies. Leukemia & lymphoma 55, 1980–1992, 10.3109/10428194.2013.855307 (2014).
Shi, L. et al. Paeoniflorin inhibits nucleus pulposus cell apoptosis by regulating the expression of Bcl-2 family proteins and caspase-9 in a rabbit model of intervertebral disc degeneration. Experimental and therapeutic medicine 10, 257–262, 10.3892/etm.2015.2501 (2015).
Zhang, X., Chen, L. X., Ouyang, L., Cheng, Y. & Liu, B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 45, 466–476, 10.1111/j.1365-2184.2012.00833.x (2012).
Aredia, F., Guaman Ortiz, L. M., Giansanti, V. & Scovassi, A. I. Autophagy and cancer. Cells 1, 520–534, 10.3390/cells1030520 (2012).
Jing, K. et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 7, 1348–1358, 10.4161/auto.7.11.16658 (2011).
Roach, P. J. AMPK -> ULK1 -> autophagy. Mol. Cell. Biol. 31, 3082–3084, 10.1128/MCB.05565-11 (2011).
Maiuri, M. C. et al. Autophagy regulation by p53. Curr. Opin. Cell Biol. 22, 181–185, 10.1016/j.ceb.2009.12.001 (2010).
Xu, D. et al. Identification and characterization of anticancer compounds targeting apoptosis and autophagy from Chinese native Garcinia species. Planta Med. 81, 79–89, 10.1055/s-0034-1383356 (2015).
Gao, H. et al. Total Tanshinones-Induced Apoptosis and Autophagy Via Reactive Oxygen Species in Lung Cancer 95D Cells. Am. J. Chin. Med., 1–15, 10.1142/S0192415X1550072X (2015).
Eisenberg-Lerner, A., Bialik, S., Simon, H. U. & Kimchi, A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 16, 966–975, 10.1038/cdd.2009.33 (2009).
Hansakul, P., Aree, K., Tanuchit, S. & Itharat, A. Growth arrest and apoptosis via caspase activation of dioscoreanone in human non-small-cell lung cancer A549 cells. BMC Complement. Altern. Med. 14, 413, 10.1186/1472-6882-14-413 (2014).
Visioli, F. et al. Glucose-regulated protein 78 (Grp78) confers chemoresistance to tumor endothelial cells under acidic stress. PLoS One 9, e101053, 10.1371/journal.pone.0101053 (2014).
Li, C. et al. Sensitization of glioma cells to tamoxifen-induced apoptosis by Pl3-kinase inhibitor through the GSK-3beta/beta-catenin signaling pathway. PLoS One 6, e27053, 10.1371/journal.pone.0027053 (2011).
Ma, Y. C. et al. Effect of Furin inhibitor on lung adenocarcinoma cell growth and metastasis. Cancer Cell Int. 14, 43, 10.1186/1475-2867-14-43 (2014).
Shen, Z. Y. et al. Autophagy and endocytosis in the amnion. J. Struct. Biol. 162, 197–204, 10.1016/j.jsb.2006.10.010 (2008).
Lao, Y. et al. The natural compound oblongifolin C inhibits autophagic flux and enhances antitumor efficacy of nutrient deprivation. Autophagy 10, 736–749, 10.4161/auto.28034 (2014).
Ren, S. X. et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One 8, e63641, 10.1371/journal.pone.0063641 (2013).
Acknowledgements
This work was supported by the NSFC Projects (No. 81373903; No. 81202946), the Key Project of Fundamental Research Fund for the Central Universities (XDJK2014B044), Chongqing Project of Science and technology talent cultivation (cstc2013kjrc-qnrc1002).
Author information
Authors and Affiliations
Contributions
H.Q. and L.L. conceived the research; H.Q., L.L. and C.L. designed the project; L.C., Z.J., H.M. and N.L. and H.C. performed the experiments; H.Q., L.L. and C.L. wrote the manuscripts; all authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, L., Jiang, Z., Ma, H. et al. Volatile Oil of Acori Graminei Rhizoma-Induced Apoptosis and Autophagy are dependent on p53 Status in Human Glioma Cells. Sci Rep 6, 21148 (2016). https://doi.org/10.1038/srep21148
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21148
This article is cited by
-
Recent insights into autophagy and metals/nanoparticles exposure
Toxicological Research (2023)
-
Molecular mechanisms of apoptosis and autophagy elicited by combined treatment with oridonin and cetuximab in laryngeal squamous cell carcinoma
Apoptosis (2019)
-
CASC2c as an unfavorable prognosis factor interacts with miR-101 to mediate astrocytoma tumorigenesis
Cell Death & Disease (2017)
-
Lithium enhances the antitumour effect of temozolomide against TP53 wild-type glioblastoma cells via NFAT1/FasL signalling
British Journal of Cancer (2017)
-
Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells
Cell Death & Disease (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.