Abstract
There are empirical indications of an isometric scaling relationship between plants’ respiratory metabolism rates and nitrogen contents. To test the hypothesis that there may be a similar relationship between plants’ respiratory metabolism and phosphorus contents we used data obtained from 150 laboratory and field-grown seedlings representing 30 herbaceous species and 20 woody deciduous species. Our results show that whole-plant respiration rates strongly scaled to the 0.81-power of the whole-plant phosphorus content, across wide ranges of growth conditions and functional classifications. Moreover, we also found a similar scaling exponent between whole-plant respiration rates and total nitrogen contents for the same set of samples. The similarities of the metabolic scaling relationships suggest that similar mechanisms may be involved in the transport and storage of phosphorus and nitrogen in plants.
Similar content being viewed by others
Introduction
Metabolic rates affect numerous (if not all) physiological and ecological processes1,2, via a general scaling relationship that can be described by the following power law equation:

Here: B is a measure of metabolic rate, such as the respiration rate; M is body mass, β is a normalization constant and α is a scaling exponent. The value of α has stimulated vigorous debate3. West et al.4,5. proposed an integrated model of plant hydrodynamics, biomechanics and branching geometry, incorporating this equation and determined α to be 0.75. Subsequently, several empirical and theoretical studies have demonstrated that the scaling exponent relating plant metabolic rates to body mass declines from nearly 1 for small seedlings and saplings to 0.75 for large plants6,7,8,9,10,11,12,13,14,15,16, due to shifts in physiological constraints on the allocation of plant biomass between photosynthetic and non-photosynthetic organs during ontogenetic progression.
As an essential component of key enzymes nitrogen is involved in crucial metabolic processes in plants and is tightly coupled with respiratory metabolism at multiple levels17,18,19,20,21. Furthermore, whole-plant respiration rates isometrically scale more consistently with total nitrogen content than with body mass22,23. Like nitrogen, phosphorus is a vital component of plants’ nucleic acids and many proteins, including enzymes involved in the respiratory release of energy contained in sugars and the regulation of numerous metabolic pathways24. Consequently, phosphorus is also considered to be a good predictor of metabolic rates in plants25,26,27 and it is required for all plant growth and development processes26,28,29. Thus, it seems reasonable to hypothesize that plants’ phosphorus contents are linked to their respiration rates through a scaling relationship similar to that observed for their nitrogen contents12,22,23,30. In the study presented here we tested this hypothesis through observations of 150 seedlings representing 30 herbaceous species and 20 deciduous woody species grown in either the laboratory or field. We measured phosphorus contents and respiration rates of the whole plants and their aboveground parts. In addition, to examine whether the hypothetical relationship (if present), is similar to that between nitrogen and respiration rates, we simultaneously measured the plants’ nitrogen contents.
Results
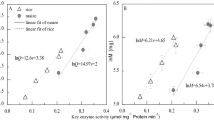
According to the pooled data for all seedlings of 50 plant species grown under greenhouse and field conditions (Table S1), the aboveground respiration rates scaled to the 0.81-power (95% CI =0.77–0.85, r2 = 0.923; P < 0.001) of the aboveground phosphorus content (Fig. 1a). Similar scaling relationships were also found for both functional groups (herbaceous and woody plants), under both greenhouse and field growth conditions (Table 1). The scaling exponent of whole-plant respiration rates to total phosphorus content (Fig. 1b) was also estimated to be 0.81 (95% CI = 0.77–0.83, r2 = 0.946; P < 0.001). Similar relationships between whole-plant respiration rates and phosphorus contents were also observed for both functional groups under both growth conditions (Table 1). Furthermore, the scaling relationships between nitrogen contents of both functional groups (either whole plants or their aboveground parts) under both growth conditions were very similar, with an estimated scaling exponent of ca. 0.82 (Fig. 1c,d; Table 2).
Log-log bivariate plots of aboveground respiration and total respiration rates (normalized to rates at 24 °C) in relation to phosphorus (a,b) and nitrogen (c,d) contents, determined from measurements of plants representing 30 herbaceous and 20 deciduous woody species grown in the field or a greenhouse, as indicated by the color-coding.
Discussion
Our measurements of 150 small laboratory- and field-grown plants of 50 species (Table S1) indicate that there is a very strong scaling relationship between plants’ respiration rates and their phosphorus contents, with a scaling exponent of 0.81 for both whole plants and their aboveground parts that is not affected by the growth conditions. Furthermore, an extremely similar scaling exponent (0.82) was found between their respiration rates and nitrogen contents.
As elements that play numerous vital structural and functional roles in plants, phosphorus and nitrogen are likely to have similar uptake, transport and allocation mechanisms for the following reasons. Both are mainly absorbed from the soil through root hairs31,32 and their further movements depend upon transport through cell membranes33. Both phosphorus and nitrogen are similarly unloaded into xylem vessels and transported upwards to the youngest leaves and other parts of the plant33,34,35. Their lateral movements in the vascular system are also similar36,37. For example, both can readily move from xylem to phloem. These observations regarding transport mechanisms suggest that both phosphorus andnitrogen contents may be constrained by the vascular distribution networks and thus have similar scaling relationships to respiration rates.
The scaling exponents for the respiration rate to nitrogen content relationships of plants we obtained differ from the 1.0-power obtained in a previous study22. However, in the cited study the biomass of the examined plants ranged from 0.01 to 1000 g while the biomass of our samples ranged from 0.1 to 200 g. Moreover, an estimated scaling exponent significantly exceeding 1.0 for the whole-plant respiration rate to total nitrogen content relationship was obtained in another study12, based on a set of samples with biomass ranging from 0.001 to 1 g. Thus, variations in the scaling exponent of respiration rates to nitrogen content may be at least partly due to variations in biomass ranges of the sampled plants. In addition, there may be considerable differences in scaling exponents between evergreen plants (which were not included in our study) and deciduous plants, because the former retain their leaves for many years and may accumulate increasing amounts of nitrogen and phosphorus, while leaves of the latter wither and are renewed annually. However, theoretical modelling indicates that the exponent of the relationship between respiration and biomass probably approaches 1.0 in small seedlings (body size < 1 g), but shifts to around 0.75 as plant biomass increases to 100 g11. The scaling exponent (0.82) of respiration to nitrogen estimated in our study based on a set of samples between 0.1 to 200 g seems to be consistent with such predictions. These findings suggest that a universal isometric relationship between whole-plant respiration rates and nitrogen content should be rejected22, but support the hypothesis that scaling relationships vary, depending on the biomasses of the examined plants8. In addition, the scaling relationship between respiration rates and phosphorus contents may vary similarly, as we discovered very similar allometric scaling relationships between respiration rates and both phosphorus and nitrogen.
Materials and Methods
Study sites
The study involved measurements of 150 plants representing 50 species of two functional groups (herbaceous and deciduous woody species; Table S1), some grown in a greenhouse at Lanzhou University’s Yuzhong Experimental Station and others collected from the field at a site on Cuiying Mountain (35.946 N, 104.137 E; Gansu Province, China), also owned by Lanzhou University.
The material grown at the Experimental Station consisted of first-year seedlings of 20 herbaceous species and 2- to 4-year-old seedlings of 12 woody species grown in a white washed greenhouse (average temperature ≈ 22 °C, mean radiation ≈ 25% of full sunlight). At Cuiying Mountain (mean annual temperature ≈ 6.3 °C), first-year seedlings of 10 naturally regenerated herbaceous species and 2- to 3-year-old saplings of eight species of woody species were sampled. For further details see Table S1.
Respiration measurements
Before measuring dark respiration rates, individual specimens were carefully dug up from the soil by hand and soil attached to the roots was washed off, to ensure that as few fine roots as possible were lost. Each entire plant was separated into aboveground parts (leaves plus stems) and belowground parts (roots), then placed in darkness for 30 min in preparation for measurement. A Li-8100 automated CO2 flux system (LI-COR, Nebraska, USA) was used to record the dark respiration rates in a customized chamber (3.5 L volume)16. Three replicate measurements were taken from three individual plants per species, each measurement lasting 5 min. The ambient temperature was recorded during all the respiration measurements. To account for temperature effects on dark respiration rates, measured rates were adjusted to corresponding rates at a standardized temperature (24 °C) using a previously published temperature-dependent Q10 model19. Whole-plant respiration rates were estimated by summing the aboveground and root respiration rates.
Phosphorus and nitrogen measurements
All of the samples of aboveground parts and roots were dried at 120 °C for 30 min, followed by 65 °C for 72 h then weighed to determine their dry weights. Dried tissue samples, including a mixture of leaves and stems (aboveground), or roots (belowground), were powdered using a mortar and pestle. The nitrogen contents of portions of the powdered samples were then analyzed using a 2400II CHNS/O Element Analyzer (Perkin-Elmer, Boston, MA, USA), with furnace temperature set at 950 °C for combustion then reduced to 640 °C. Phosphorus contents of portions of the powdered above- and below-ground tissue samples were also analyzed, using the molybdate/ascorbic acid method after H2SO4–H2O2 digestion38. The nitrogen and phosphorus contents of the aboveground parts and roots of the sampled plants were then calculated by multiplying their measured nitrogen and phosphorus concentrations (g g−1) and dry biomasses (g). The total plant nitrogen and phosphorus contents were calculated by simply summing the aboveground (leaves and stems) and belowground (roots) contents.
Statistical analysis
All data were log10-transformed to allow expression of the power function in the form of a linear regression equation, which was used to estimate parameters for each variable and confidence intervals for the parameters39,40. Type II (reduced major axis) regression models were used to determine scaling exponents (α) and normalization constants (β) from the log10-transformed data using SMATR Version 2.041,42.
Additional Information
How to cite this article: Wang, Z.-Q. et al. Scaling the respiratory metabolism to phosphorus relationship in plant seedlings. Sci. Rep. 5, 16377; doi: 10.1038/srep16377 (2015).
References
Reich, P. B. et al. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: a test across biomes and functional groups. Oecologia 114, 471–482 (1998).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Towards a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Dodds, P. S., Rothman, D. H. & Weitz, J. S. Re-examination of the ‘3/4-law’ of metabolism. J. Theor. Biol. 209, 9–27 (2001).
West, G. B., Brown, J. H. & Enquist, B. J. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (1997).
West, G. B., Brown, J. H. & Enquist, B. J. A general model for the structure and allometry of plant vascular systems. Nature 400, 664–667 (1999).
Niklas, K. J. & Enquist, B. J. On the vegetative biomass partitioning of seed plant leaves, stem and roots. Am. Nat. 159, 482–497 (2002).
Niklas, K. J. Plant allometry: is there a grand unifying theory? Biol. Rev. Camb. Philos. Soc. 79, 871–889 (2004).
Enquist, B. J. et al. Biological scaling: does the exception prove the rule? Nature 445, E9–E10 (2007).
Price, C. A., Enquist, B. J. & Savage, V. M. A general model for allometric covariation in botanical form and function. Proc. Natl. Acad. Sci. USA 104, 204–209 (2007).
Cheng, D. L., Li, T., Zhong, Q. L. & Wang, G. X. Scaling relationship between tree respiration rates and biomass. Biol. Lett. 6, 715–717 (2010).
Mori, S. et al. Mixed-power scaling of whole-plant respiration from seedlings to giant trees. Proc. Natl. Acad. Sci. USA 107, 1447–1451 (2010).
Peng, Y. H., Niklas, K. J., Reich, P. B. & Sun, S. C. Ontogenetic shift in the scaling of dark respiration with whole-plant mass in seven shrub species. Funct. Ecol. 24, 502–512 (2010).
Deng, J. M. et al. Models and tests of optimal density and maximal yield for crop plants. Proc. Natl. Acad. Sci. USA 109, 15823–15828 (2012a).
Deng, J. M. et al. Insights into plant size-density relationships from models and agricultural crops. Proc. Natl. Acad. Sci. USA 109, 8600–8605 (2012b).
Cheng, D. L., Niklas, K. J., Zhong, Q. L., Yang, Y. S. & Zhang, J. H. Interspecific differences in whole-plant respiration vs.biomass scaling relationships: A case study using evergreen conifer and angiosperm tree seedlings. Am. J. Bot. 101, 617–623 (2014).
Wang, Z. Q. et al. A theoretical framework for whole-plant carbon assimilation efficiency based on metabolic scaling theory: a test case using Picea seedling. Tree Physiol. 35, 599–607 (2015).
Ryan, M. G. Foliar maintenance respiration of subalpine and boreal trees and shrubs in relation to nitrogen content. Plant Cell Environ. 18, 765–772 (1995).
Reich, P.B., Walters, M.B., Tjoelker, M.G., Vanderklein, D. & Bushena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 12, 395–405 (1998).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Atkinson, L. J., Hellicar, M. A., Fitter, A. H. & Atkin, O. K. Impact of temperature on the relationship between respiration and nitrogen concentration in roots: an analysis of scaling relationships, Q10 values and thermal acclimation ratios. New Phytol. 173, 110–120 (2007).
Machado, J. L. & Reich, P. B. Dark respiration rate increases with plant size in saplings of three temperate tree species despite decreasing tissue nitrogen and nonstructural carbohydrates. Tree Physiol. 26, 915–923 (2006).
Reich, P. B., Tjoelker, M. G., Machado, J. L. & Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439, 457–461 (2006).
Reich, P. B., Tjoelker, M. G., Machada, J. L. & Oleksyn, J. Biological scaling: does the exception prove the rule? (Reply) Nature 445, E10–E11 (2007).
Theodorou, M. E. & Plaxton, W. C. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 101, 339–344 (1993).
Hedin, L. O. Physiology: Plants on a different scale. Nature 439, 399–400 (2006).
Sterner, R. W. & Elser, J. J. Ecological stoichiometry-the biology of elements from molecules to the biosphere (Princeton University Press, Princeton, NJ, 2002).
Elser, J. J. et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943 (2003).
Atkin, O. K. & Tjoelker, M. G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 8, 343–351 (2003).
Ågren, G. I. Stoichiometry and nutrition of plant growth in natural communities. Ann. Rev. Ecol. Syst. 39, 153–170 (2008).
Zhang, Q. The allometric scaling law: plant photosynthesis, respiration, in relation to plant body size and nitrogen content. PhD dissertation, Lanzhou University (2011).
Lynch, J. Root architecture and plant productivity. Plant Physiol. 109, 7–13 (1995).
Schachtman, D. P., Reid, R. J. & Ayling, S. M. Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116, 447–453 (1998).
Mimura, T. Homeostasis and transport of inorganic phosphate in plants. Plant Cell Physiol. 36, 1–7 (1995).
Jeschke, W., Kirkby, E., Peuke, A., Pate, J. & Hartung, W. Effects of P efficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). J. Exp. Bot. 48, 75–91 (1997).
Aibara, I. & Miwa, K. Strategies for optimization of mineral nutrient transport in plants: multilevel regulation of nutrient-dependent dynamics of root architecture and transporter activity. Plant Cell Physiol. 55, 2027–2036 (2014).
Bieleski, R. L. Phosphate pools, phosphate transport and phosphate availability. Annu. Rev. Plant Physiol. 24, 225–252 (1973).
Hill, J. Remobilization of nutrients from leaves. J. Plant Nutr. 2, 407–444 (1980).
Jones, J. B. Laboratory guide for conducting soil tests and plant analysis (CRC Press, New York, 2001).
Freckleton, R. P. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 71, 1367–1375 (2002).
Downs, C. J., Hayes, J. P. & Tracy, C. R. Scaling metabolic rate with body mass and inverse body temperature: a test of the Arrhenius fractal supply model. Funct. Ecol. 22, 239–244 (2008).
Falster, D. S., Warton, D. I. & Wright, I. J. User’s guide to SMATR: Standardised Major Axis Test & Routines Version 2.0, Copyright 2006. http://www.bio.mq.edu.au/ecology/SMATR/11 March 2006 (2006).
Warton, D. I., Wright, I. J., Falster, D. S. & Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 81, 259–291 (2006).
Acknowledgements
We thank Jiangtao Li and Xiaowei Li for their assistance in the laboratory and Lixin Qiao for identifying plant species in the field. The research was supported by grants from the National Key Project for Basic Research (2014CB954100), Sichuan Province Youth Science and Technology Innovation Team (2014TD003), National Natural Science Foundation of China and postdoctoral fund of Sichuan University the China Postdoctoral Science Foundation funded project (2015M582547).
Author information
Authors and Affiliations
Contributions
Z.Q.W. and H.H. carried out the experimental work and participated inthe data analysis. Z.Q.W. drafted and J.Q.L. revised, the manuscript. Z.Q.W., J.M.D. and J.Q.L. designed and coordinated the study. All authors gave final approval for publication. We thank John Blackwell from Sees-ltd to improve English of the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, ZQ., Huang, H., Deng, JM. et al. Scaling the respiratory metabolism to phosphorus relationship in plant seedlings. Sci Rep 5, 16377 (2015). https://doi.org/10.1038/srep16377
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16377
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.